Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways
- PMID: 20008023
- PMCID: PMC2840852
- DOI: 10.1210/jc.2009-1888
Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways
Abstract
Context: Radioiodine ablation is commonly used to treat thyroid cancer, but a major challenge is often the loss of radioiodine avidity of the cancer caused by aberrant silencing of iodide-handling genes.
Objectives: This study was conducted to test the therapeutic potential of targeting the aberrantly activated MAPK and PI3K/Akt/mTOR pathways and histone deacetylase to restore radioiodine avidity in thyroid cancer cells.
Experimental design: We tested the effects of specific inhibitors targeting these pathways/molecules that had established clinical applicability, including the MAPK kinase inhibitor RDEA119, mTOR inhibitor temsirolimus, Akt inhibitor perifosine, and histone deacetylase inhibitor SAHA, individually or in combinations, on the expression of iodide-handling genes and radioiodide uptake in a large panel of thyroid cancer cell lines.
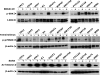
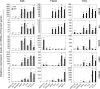
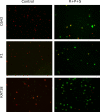
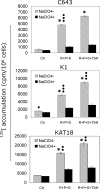
Results: The expression of a large number of iodide-handling genes could be restored, particularly the sodium/iodide symporter, TSH receptor, and thyroperoxidase, by treating cells with these inhibitors. The effect was particularly robust and synergistic when combinations of inhibitors containing SAHA were used. Robust expression of sodium/iodide symporter in the cell membrane, which plays the most important role in iodide uptake in thyroid cells, was confirmed by immunofluorescent microscopy. Radioiodide uptake by cells was correspondingly induced under these conditions. Thyroid gene expression and radioiodide uptake could both be further enhanced by TSH.
Conclusions: Targeting major signaling pathways could restore thyroid gene expression and radioiodide uptake in thyroid cancer cells. Further studies are warranted to test this therapeutic potential in restoring radioiodine avidity of thyroid cancer cells for effective ablation treatment.
Figures

Similar articles
-
Induction of sodium/iodide symporter (NIS) expression and radioiodine uptake in non-thyroid cancer cells.PLoS One. 2012;7(2):e31729. doi: 10.1371/journal.pone.0031729. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359623 Free PMC article.
-
Robust Thyroid Gene Expression and Radioiodine Uptake Induced by Simultaneous Suppression of BRAF V600E and Histone Deacetylase in Thyroid Cancer Cells.J Clin Endocrinol Metab. 2016 Mar;101(3):962-71. doi: 10.1210/jc.2015-3433. Epub 2016 Jan 11. J Clin Endocrinol Metab. 2016. PMID: 26751190 Free PMC article.
-
MTOR downregulates iodide uptake in thyrocytes.J Endocrinol. 2010 Jul;206(1):113-20. doi: 10.1677/JOE-09-0436. Epub 2010 Apr 14. J Endocrinol. 2010. PMID: 20392814
-
Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS.Theranostics. 2021 Apr 15;11(13):6251-6277. doi: 10.7150/thno.57689. eCollection 2021. Theranostics. 2021. PMID: 33995657 Free PMC article. Review.
-
Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism.Eur J Endocrinol. 1999 Nov;141(5):443-57. doi: 10.1530/eje.0.1410443. Eur J Endocrinol. 1999. PMID: 10576759 Review.
Cited by
-
Induction of sodium/iodide symporter (NIS) expression and radioiodine uptake in non-thyroid cancer cells.PLoS One. 2012;7(2):e31729. doi: 10.1371/journal.pone.0031729. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359623 Free PMC article.
-
Molecular pathogenesis and mechanisms of thyroid cancer.Nat Rev Cancer. 2013 Mar;13(3):184-99. doi: 10.1038/nrc3431. Nat Rev Cancer. 2013. PMID: 23429735 Free PMC article. Review.
-
The sodium iodide symporter (NIS) as an imaging reporter for gene, viral, and cell-based therapies.Curr Gene Ther. 2012 Feb 1;12(1):33-47. doi: 10.2174/156652312799789235. Curr Gene Ther. 2012. PMID: 22263922 Free PMC article. Review.
-
PDGFRα Regulates Follicular Cell Differentiation Driving Treatment Resistance and Disease Recurrence in Papillary Thyroid Cancer.EBioMedicine. 2016 Oct;12:86-97. doi: 10.1016/j.ebiom.2016.09.007. Epub 2016 Sep 10. EBioMedicine. 2016. PMID: 27682510 Free PMC article.
-
Emerging strategies for managing differentiated thyroid cancers refractory to radioiodine.Endocrine. 2016 May;52(2):214-21. doi: 10.1007/s12020-015-0830-4. Epub 2015 Dec 21. Endocrine. 2016. PMID: 26690657 Review.
References
-
- Leenhardt L, Grosclaude P, Chérié-Challine L 2004 Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid 14:1056–1060 - PubMed
-
- Davies L, Welch HG 2006 Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ 2009 Cancer statistics, 2009. CA Cancer J Clin 59:225–249 - PubMed
-
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM 2009 American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 - PubMed
-
- Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W 2006 European Thyroid Cancer Taskforce. European Consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 154:787–803 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

