The cyclic nucleotide monophosphate domain of Xanthomonas campestris global regulator Clp defines a new class of cyclic di-GMP effectors
- PMID: 20008070
- PMCID: PMC2812978
- DOI: 10.1128/JB.01253-09
The cyclic nucleotide monophosphate domain of Xanthomonas campestris global regulator Clp defines a new class of cyclic di-GMP effectors
Abstract
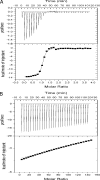
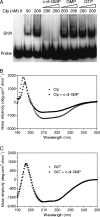
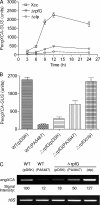
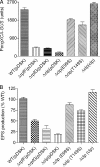
The widely conserved second messenger cyclic diguanosine monophosphate (c-di-GMP) plays a key role in quorum-sensing (QS)-dependent production of virulence factors in Xanthomonas campestris pv. campestris. The detection of QS diffusible signal factor (DSF) by the sensor RpfC leads to the activation of response regulator RpfG, which activates virulence gene expression by degrading c-di-GMP. Here, we show that a global regulator in the X. campestris pv. campestris QS regulatory pathway, Clp, is a c-di-GMP effector. c-di-GMP specifically binds to Clp with high affinity and induces allosteric conformational changes that abolish the interaction between Clp and its target gene promoter. Clp is similar to the cyclic AMP (cAMP) binding proteins Crp and Vfr and contains a conserved cyclic nucleotide monophosphate (cNMP) binding domain. Using site-directed mutagenesis, we found that the cNMP binding domain of Clp contains a glutamic acid residue (E99) that is essential for c-di-GMP binding. Substituting the residue with serine (E99S) resulted in decreased sensitivity to changes in the intracellular c-di-GMP level and attenuated bacterial virulence. These data establish the direct role of Clp in the response to fluctuating c-di-GMP levels and depict a novel mechanism by which QS links the second messenger with the X. campestris pv. campestris virulence regulon.
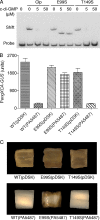
Figures


References
-
- Amikam, D., and M. Y. Galperin. 2006. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 22:3-6. - PubMed
-
- Christen, B., M. Christen, R. Paul, F. Schmid, M. Folcher, P. Jenoe, M. Meuwly, and U. Jenal. 2006. Allosteric control of cyclic di-GMP signaling. J. Biol. Chem. 281:32015-32024. - PubMed
-
- Dow, J. M., Y. Fouhy, J. F. Lucey, and R. P. Ryan. 2006. The HD-GYP domain, cyclic di-GMP signaling, and bacterial virulence to plants. Mol. Plant Microbe Interact. 19:1378-1384. - PubMed
-
- Gough, C. L., J. M. Dow, J. Keen, B. Henrissat, and M. J. Daniels. 1990. Nucleotide sequence of the engXCA gene encoding the major endoglucanase of Xanthomonas campestris pv. campestris. Gene 89:53-59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

