Temporal and spatial regulation of the four transcription start sites of hetR from Anabaena sp. strain PCC 7120
- PMID: 20008074
- PMCID: PMC2812969
- DOI: 10.1128/JB.01297-09
Temporal and spatial regulation of the four transcription start sites of hetR from Anabaena sp. strain PCC 7120
Abstract
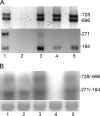
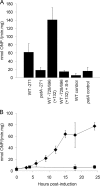
The filamentous cyanobacterium Anabaena sp. strain PCC 7120 forms nitrogen-fixing heterocysts in a periodic pattern in response to combined-nitrogen limitation in the environment. The master regulator of heterocyst differentiation, HetR, is necessary for both pattern formation and commitment of approximately every 10th cell of a filament to differentiation into a heterocyst. In this study, the individual contributions of four transcriptional start points (tsps) in regulation of transcription of hetR were assessed, and the effects of the regulatory genes patS, hetN, and patA on transcription from the tsps were determined. The tsp located at nucleotide -271 relative to the translational start site (-271 tsp) was the most tightly regulated tsp, and fluorescence from a -271 tsp-green fluorescent protein (GFP) reporter fusion was observed initially in groups of two cells and later in single cells arranged in a spatial pattern that mimicked the pattern of heterocysts that emerged. Conversely, the fluorescence from the -184 and -728/-696 tsp-GFP reporter fusions was uniform throughout filaments. Transcription from the -271 tsp was severely downregulated in a strain in which the patA gene, which encodes a positive regulator of differentiation, was deleted, and it was not detectable in strains overexpressing the genes encoding the negative regulators of differentiation, patS and hetN. In strains lacking the -271 tsp of hetR, pattern formation, the timing of commitment to differentiation, and the number of cells that differentiated into heterocysts were affected. Taken together, these results demonstrate the role of regulation of the -271 tsp of hetR in the genetic network that governs heterocyst pattern formation and differentiation.
Figures



Similar articles
-
Transcriptional regulation of the heterocyst patterning gene patA from Anabaena sp. strain PCC 7120.J Bacteriol. 2010 Sep;192(18):4732-40. doi: 10.1128/JB.00577-10. Epub 2010 Jul 9. J Bacteriol. 2010. PMID: 20622060 Free PMC article.
-
Epistasis analysis of four genes from Anabaena sp. strain PCC 7120 suggests a connection between PatA and PatS in heterocyst pattern formation.J Bacteriol. 2006 Mar;188(5):1808-16. doi: 10.1128/JB.188.5.1808-1816.2006. J Bacteriol. 2006. PMID: 16484191 Free PMC article.
-
HetF and PatA control levels of HetR in Anabaena sp. strain PCC 7120.J Bacteriol. 2008 Dec;190(23):7645-54. doi: 10.1128/JB.01110-08. Epub 2008 Oct 3. J Bacteriol. 2008. PMID: 18835986 Free PMC article.
-
cyAbrB Transcriptional Regulators as Safety Devices To Inhibit Heterocyst Differentiation in Anabaena sp. Strain PCC 7120.J Bacteriol. 2019 Aug 8;201(17):e00244-19. doi: 10.1128/JB.00244-19. Print 2019 Sep 1. J Bacteriol. 2019. PMID: 31085690 Free PMC article.
-
Heterocyst differentiation and pattern formation in cyanobacteria: a chorus of signals.Mol Microbiol. 2006 Jan;59(2):367-75. doi: 10.1111/j.1365-2958.2005.04979.x. Mol Microbiol. 2006. PMID: 16390435 Review.
Cited by
-
HetL, HetR and PatS form a reaction-diffusion system to control pattern formation in the cyanobacterium nostoc PCC 7120.Elife. 2020 Aug 7;9:e59190. doi: 10.7554/eLife.59190. Elife. 2020. PMID: 32762845 Free PMC article.
-
R-DeeP/TripepSVM identifies the RNA-binding OB-fold-like protein PatR as regulator of heterocyst patterning.Nucleic Acids Res. 2025 Jan 24;53(3):gkae1247. doi: 10.1093/nar/gkae1247. Nucleic Acids Res. 2025. PMID: 39698830 Free PMC article.
-
Regulatory RNAs in photosynthetic cyanobacteria.FEMS Microbiol Rev. 2015 May;39(3):301-15. doi: 10.1093/femsre/fuv017. Epub 2015 Apr 30. FEMS Microbiol Rev. 2015. PMID: 25934122 Free PMC article. Review.
-
Structure of transcription factor HetR required for heterocyst differentiation in cyanobacteria.Proc Natl Acad Sci U S A. 2011 Jun 21;108(25):10109-14. doi: 10.1073/pnas.1106840108. Epub 2011 May 31. Proc Natl Acad Sci U S A. 2011. PMID: 21628585 Free PMC article.
-
Comparative analysis of the primary transcriptome of Synechocystis sp. PCC 6803.DNA Res. 2014 Oct;21(5):527-39. doi: 10.1093/dnares/dsu018. Epub 2014 Jun 16. DNA Res. 2014. PMID: 24935866 Free PMC article.
References
-
- Black, T. A., Y. Cai, and C. P. Wolk. 1993. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 9:77-84. - PubMed
-
- Borthakur, P. B., C. C. Orozco, S. S. Young-Robbins, R. Haselkorn, and S. M. Callahan. 2005. Inactivation of patS and hetN causes lethal levels of heterocyst differentiation in the filamentous cyanobacterium Anabaena sp. PCC 7120. Mol. Microbiol. 57:111-123. - PubMed
-
- Buikema, W. J., and R. Haselkorn. 1991. Characterization of a gene controlling heterocyst development in the cyanobacterium Anabaena 7120. Genes Dev. 5:321-330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

