Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice
- PMID: 20008116
- PMCID: PMC2838674
- DOI: 10.1152/ajplung.00089.2009
Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice
Abstract
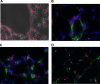
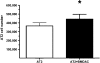
Neonatal hyperoxia impairs vascular and alveolar growth in mice and decreases endothelial progenitor cells. To determine the role of bone marrow-derived cells in restoration of neonatal lung structure after injury, we studied a novel bone marrow myeloid progenitor cell population from Tie2-green fluorescent protein (GFP) transgenic mice (bone marrow-derived angiogenic cells; BMDAC). We hypothesized that treatment with BMDAC would restore normal lung structure in infant mice during recovery from neonatal hyperoxia. Neonatal mice (1-day-old) were exposed to 80% oxygen for 10 days. BMDACs (1 x 10(5)), embryonic endothelial progenitor cells, mouse embryonic fibroblasts (control), or saline were then injected into the pulmonary circulation. At 21 days of age, saline-treated mice had enlarged alveoli, reduced septation, and a reduction in vascular density. In contrast, mice treated with BMDAC had complete restoration of lung structure that was indistinguishable from room air controls. BMDAC comprised 12% of distal lung cells localized to pulmonary vessels or alveolar type II (AT2) cells and persist (8.8%) for 8 wk postinjection. Coculture of AT2 cells or lung endothelial cells (luEC) with BMDAC augmented AT2 and luEC cell growth in vitro. We conclude that treatment with BMDAC after neonatal hyperoxia restores lung structure in this model of bronchopulmonary dysplasia.
Figures




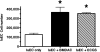
References
-
- Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, Wang X, Hashimoto M, Sharp JG, Rennard SI. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med 170: 1158–1163, 2004 - PubMed
-
- Abman S. Pulmonary hypertension in chronic lung disease of infancy: pathogenesis, pathophysiology, and treatment. In: Chronic Lung Disease in Early Infancy, edited by Bland RD, Coalson JJ. New York: Dekker, 1999, p. 619–668
-
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228, 1999 - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

