Bile acids: analysis in biological fluids and tissues
- PMID: 20008121
- PMCID: PMC2789783
- DOI: 10.1194/jlr.R001941-JLR200
Bile acids: analysis in biological fluids and tissues
Abstract
The formation of bile acids/bile alcohols is of major importance for the maintenance of cholesterol homeostasis. Besides their functions in lipid absorption, bile acids/bile alcohols are regulatory molecules for a number of metabolic processes. Their effects are structure-dependent, and numerous metabolic conversions result in a complex mixture of biologically active and inactive forms. Advanced methods are required to characterize and quantify individual bile acids in these mixtures. A combination of such analyses with analyses of the proteome will be required for a better understanding of mechanisms of action and nature of endogenous ligands. Mass spectrometry is the basic detection technique for effluents from chromatographic columns. Capillary liquid chromatography-mass spectrometry with electrospray ionization provides the highest sensitivity in metabolome analysis. Classical gas chromatography-mass spectrometry is less sensitive but offers extensive structure-dependent fragmentation increasing the specificity in analyses of isobaric isomers of unconjugated bile acids. Depending on the nature of the bile acid/bile alcohol mixture and the range of concentration of individuals, different sample preparation sequences, from simple extractions to group separations and derivatizations, are applicable. We review the methods currently available for the analysis of bile acids in biological fluids and tissues, with emphasis on the combination of liquid and gas phase chromatography with mass spectrometry.
Figures


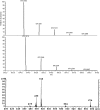

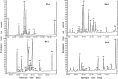
References
-
- Motola D. L., Cummins C. L., Rottiers V., Sharma K. K., Li T., Li Y., Suino-Powell K., Xu H. E., Auchus R. J., Antebi A., et al. 2006. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 124: 1209–1223. - PubMed
-
- Maneerat S., Nitoda T., Kanzaki H., Kawai F. 2005. Bile acids are new products of a marine bacterium, Myroides sp. strain SM1. Appl. Microbiol. Biotechnol. 67: 679–683. - PubMed
-
- Sobotka H. (1937) Physiological chemistry of the bile. Ballière, Tyndall & Cox, London.
-
- Sobotka H. (1938) Chemistry of the sterids. Ballière, Tyndall & Cox, London.
-
- Bergström S., Danielsson H., Samuelsson B. (1960) Formation and metabolism of bile acids. Lipide Metabolism. Block K., editor John Wiley & Sons, New York: 291–366.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

