Antigen-specific induction of osteopontin contributes to the chronification of allergic contact dermatitis
- PMID: 20008129
- PMCID: PMC2797887
- DOI: 10.2353/ajpath.2010.090488
Antigen-specific induction of osteopontin contributes to the chronification of allergic contact dermatitis
Abstract
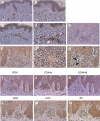
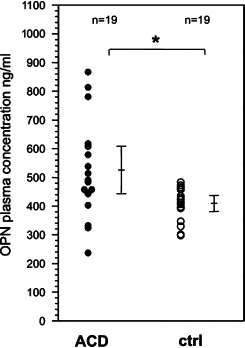
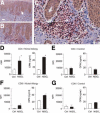
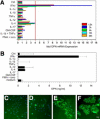
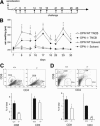
Allergic contact dermatitis is a T cell-mediated immune response, which in its relapsing chronic form is of high socioeconomic impact. The phosphoglycoprotein osteopontin (OPN) has chemotactic and Th1 cytokine functions and in various models is essential for robust T cell-mediated immunity. Here we demonstrate that OPN is abundantly expressed by both effector T cells and keratinocytes in allergic contact dermatitis lesions. T cells from nickel-allergic donors secrete high levels of OPN following antigen-specific stimulation. OPN may substitute for missing IFN-gamma secretion in T effector cells because low IFN-gamma-producing T cell clones secrete high levels of OPN, and OPN down-modulates their interleukin-4 expression. Furthermore, interferon-gamma from T effector cells augments OPN in allergic contact dermatitis by inducing OPN in keratinocytes, which in turn polarizes dendritic cells and attracts inflammatory cells. In the murine contact hypersensitivity (CHS) model for allergic contact dermatitis, OPN is strongly induced in antigen-specific proliferating T cells, and OPN null mice display a reduced chronic CHS inflammatory response due to a decreased influx of effector T cells. Importantly, because of its function for chronic allergic contact dermatitis, OPN may well be a therapeutic target, because anti-OPN antibody treatment in part suppresses established chronic CHS.
Figures

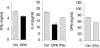
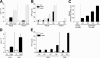


References
-
- Diepgen TL, Coenraads PJ. The epidemiology of occupational contact dermatitis. Int Arch Occup Environ Health. 1999;72:496–506. - PubMed
-
- Kimber I, Dearman RJ. Allergic contact dermatitis: the cellular effectors. Contact Dermatitis. 2002;46:1–5. - PubMed
-
- Cavani A, Girolomoni G. Immune mechanisms in allergic contact dermatitis. Landes Bioscience; Georgetown, TX: 2005. p. 1.
-
- Hogan DJ, Dannaker CJ, Maibach HI. The prognosis of contact dermatitis. J Am Acad Dermatol. 1990;23:300–307. - PubMed
-
- Thyssen JP, Johansen JD, Menne T. Contact allergy epidemics and their controls. Contact Dermatitis. 2007;56:185–195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

