Initiation of acquired immunity in the lungs of mice lacking lymph nodes after infection with aerosolized Mycobacterium tuberculosis
- PMID: 20008132
- PMCID: PMC2797882
- DOI: 10.2353/ajpath.2010.090446
Initiation of acquired immunity in the lungs of mice lacking lymph nodes after infection with aerosolized Mycobacterium tuberculosis
Abstract
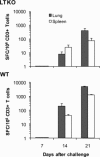
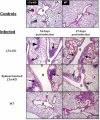
Recent evidence points to lung draining lymph nodes as the site that initiates the immune response in mice infected with aerosolized Mycobacterium tuberculosis. Here we expanded these studies and showed that infection of mice that lack lymph nodes with aerosolized M. tuberculosis results in a massive mononuclear cell infiltrate in the lungs within 14 days postinfection. This infiltration clearly resembles an expansion of the bronchus-associated lymphoid tissue. As expected, no bronchus-associated lymphoid tissue was observed in M. tuberculosis-infected wild-type control mice. Importantly, acquired specific immune response to M. tuberculosis antigens could be detected in lung lymphocytes harvested from mice lacking lymph nodes as early as 14 days postinfection. In addition, the bacterial burden in these mice was indistinguishable from that observed in wild-type C57BL/6 control mice. These results indicate that in the absence of lymph nodes, priming of the immune response occurs in the lung tissues after infection of mice with aerosolized M. tuberculosis and clearly illustrate the enormous plasticity of the immune system to develop resistance to foreign pathogens.
Figures

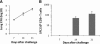

Similar articles
-
CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages.J Immunol. 2000 Jul 1;165(1):353-63. doi: 10.4049/jimmunol.165.1.353. J Immunol. 2000. PMID: 10861072
-
The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes.J Immunol. 2003 May 15;170(10):5210-8. doi: 10.4049/jimmunol.170.10.5210. J Immunol. 2003. PMID: 12734369
-
Selectin ligand-independent priming and maintenance of T cell immunity during airborne tuberculosis.J Immunol. 2006 Jan 15;176(2):1131-40. doi: 10.4049/jimmunol.176.2.1131. J Immunol. 2006. PMID: 16394002
-
Orchestration of pulmonary T cell immunity during Mycobacterium tuberculosis infection: immunity interruptus.Semin Immunol. 2014 Dec;26(6):559-77. doi: 10.1016/j.smim.2014.09.003. Epub 2014 Oct 11. Semin Immunol. 2014. PMID: 25311810 Free PMC article. Review.
-
T cell-mediated host immune defenses in the lung.Annu Rev Immunol. 2013;31:605-33. doi: 10.1146/annurev-immunol-032712-100019. Annu Rev Immunol. 2013. PMID: 23516986 Free PMC article. Review.
Cited by
-
Mucosal delivery of tuberculosis vaccines: a review of current approaches and challenges.Expert Rev Vaccines. 2019 Dec;18(12):1271-1284. doi: 10.1080/14760584.2019.1692657. Epub 2019 Dec 26. Expert Rev Vaccines. 2019. PMID: 31876199 Free PMC article. Review.
-
Local Pulmonary Immunological Biomarkers in Tuberculosis.Front Immunol. 2021 Mar 5;12:640916. doi: 10.3389/fimmu.2021.640916. eCollection 2021. Front Immunol. 2021. PMID: 33746984 Free PMC article. Review.
-
Bronchus-associated lymphoid tissue (BALT) structure and function.Adv Immunol. 2010;107:187-241. doi: 10.1016/B978-0-12-381300-8.00007-1. Adv Immunol. 2010. PMID: 21034975 Free PMC article. Review.
-
An unbiased peptide-wide discovery approach to select Mycobacterium tuberculosis antigens that target CD8+ T cell response during infection.Vaccine. 2013 Oct 1;31(42):4834-40. doi: 10.1016/j.vaccine.2013.07.077. Epub 2013 Aug 9. Vaccine. 2013. PMID: 23933335 Free PMC article.
-
Mycobacteria-Specific T Cells Are Generated in the Lung During Mucosal BCG Immunization or Infection With Mycobacterium tuberculosis.Front Immunol. 2020 Oct 22;11:566319. doi: 10.3389/fimmu.2020.566319. eCollection 2020. Front Immunol. 2020. PMID: 33193338 Free PMC article.
References
-
- Stewart GR, Robertson BD, Young DB. Tuberculosis: a problem with persistence. Nat Rev Microbiol. 2003;1:97–105. - PubMed
-
- Medlar EM. Pathogenetic concepts of tuberculosis. Am J Med. 1950;9:611–622. - PubMed
-
- Lurie MB. Native and acquired resistance to tuberculosis. Am J Med. 1950;9:591–610. - PubMed
-
- Perlman DC, El-Helou P, Salomon N. Tuberculosis in patients with human immunodeficiency virus infection. Semin Respir Infect. 1999;14:344–352. - PubMed
-
- Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis. 2003;3:578–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

