Endometrial cancer side-population cells show prominent migration and have a potential to differentiate into the mesenchymal cell lineage
- PMID: 20008133
- PMCID: PMC2797898
- DOI: 10.2353/ajpath.2010.090056
Endometrial cancer side-population cells show prominent migration and have a potential to differentiate into the mesenchymal cell lineage
Abstract
Cancer stem-like cell subpopulations, referred to as "side-population" (SP) cells, have been identified in several tumors based on their ability to efflux the fluorescent dye Hoechst 33342. Although SP cells have been identified in the normal human endometrium and endometrial cancer, little is known about their characteristics. In this study, we isolated and characterized the SP cells in human endometrial cancer cells and in rat endometrial cells expressing oncogenic human K-Ras protein. These SP cells showed i) reduction in the expression levels of differentiation markers; ii) long-term proliferative capacity of the cell cultures; iii) self-renewal capacity in vitro; iv) enhancement of migration, lamellipodia, and uropodia formation; and v) enhanced tumorigenicity. In nude mice, SP cells formed large, invasive tumors, which were composed of both tumor cells and stromal-like cells with enriched extracellular matrix. The expression levels of vimentin, alpha-smooth muscle actin, and collagen III were enhanced in SP tumors compared with the levels in non-SP tumors. In addition, analysis of microdissected samples and fluorescence in situ hybridization of Hec1-SP-tumors showed that the stromal-like cells with enriched extracellular matrix contained human DNA, confirming that the stromal-like cells were derived from the inoculated cells. Moreober, in a Matrigel assay, SP cells differentiated into alpha-smooth muscle actin-expressing cells. These findings demonstrate that SP cells have cancer stem-like cell features, including the potential to differentiate into the mesenchymal cell lineage.
Figures
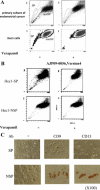



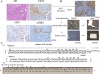


Comment in
-
Endometrial cells get side-tracked: side population cells promote epithelial-mesenchymal transition in endometrial carcinoma.Am J Pathol. 2010 Jan;176(1):25-8. doi: 10.2353/ajpath.2010.090775. Epub 2009 Nov 30. Am J Pathol. 2010. PMID: 19948831 Free PMC article.
References
-
- Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. - PubMed
-
- Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther. 2002;9:642–647. - PubMed
-
- Spangrude GJ, Smith L, Uchida N, Ikuta K, Heimfeld S, Friedman J, Weissman IL. Mouse hematopoietic stem cells. Blood. 1991;78:1395–1402. - PubMed
-
- Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

