Venular basement membranes ubiquitously express matrix protein low-expression regions: characterization in multiple tissues and remodeling during inflammation
- PMID: 20008148
- PMCID: PMC2797906
- DOI: 10.2353/ajpath.2010.090510
Venular basement membranes ubiquitously express matrix protein low-expression regions: characterization in multiple tissues and remodeling during inflammation
Abstract
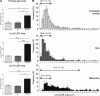
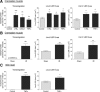
The venular basement membrane plays a critical role in maintaining the integrity of blood vessels and through its dense and highly organized network of matrix proteins also acts as a formidable barrier to macromolecules and emigrating leukocytes. Leukocytes can however penetrate the venular basement membrane at sites of inflammation, though the associated in vivo mechanisms are poorly understood. Using whole mount immunostained tissues and confocal microscopy, we demonstrate that the venular basement membrane of multiple organs expresses regions of low matrix protein (laminin-511 and type IV collagen) deposition that have been termed low-expression regions (LERs). In the multiple tissues analyzed (eg, cremaster muscle, skin, mesenteric tissue), LERs were directly aligned with gaps between adjacent pericytes and were more prevalent in small venules. As predicted by their permissive nature, LERs acted as "gates" for transmigrating neutrophils in all inflammatory reactions investigated (elicited by leukotriene B(4) [LTB(4)], CXCL1, tumor necrosis factor [TNF]alpha, endotoxin, and ischemia/reperfusion [I/R] injury), and this response was associated with an enhancement of the size of laminin-511 and type IV collagen LERs. Transmigrated neutrophils stained positively for laminins but not type IV collagen, suggesting that different mechanisms exist in remodeling of different basement membrane networks. Collectively the findings provide further insight into characteristics of specialized regions within venular basement membranes that are preferentially used and remodeled by transmigrating neutrophils.
Figures




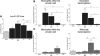


References
-
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. - PubMed
-
- Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. - PubMed
-
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der MK, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. - PubMed
-
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. - PubMed
-
- Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

