Drug-induced thrombocytopenia: pathogenesis, evaluation, and management
- PMID: 20008194
- PMCID: PMC4413903
- DOI: 10.1182/asheducation-2009.1.153
Drug-induced thrombocytopenia: pathogenesis, evaluation, and management
Abstract
Although drugs are a common cause of acute immune-mediated thrombocytopenia in adults, the drug etiology is often initially unrecognized. Most cases of drug-induced thrombocytopenia (DITP) are caused by drug-dependent antibodies that are specific for the drug structure and bind tightly to platelets by their Fab regions but only in the presence of the drug. A comprehensive database of 1301 published reports describing 317 drugs, available at www.ouhsc.edu/platelets, provides information on the level of evidence for a causal relation to thrombocytopenia. Typically, DITP occurs 1 to 2 weeks after beginning a new drug or suddenly after a single dose when a drug has previously been taken intermittently. However, severe thrombocytopenia can occur immediately after the first administration of antithrombotic agents that block fibrinogen binding to platelet GP IIb-IIIa, such as abciximab, tirofiban, and eptifibatide. Recovery from DITP usually begins within 1 to 2 days of stopping the drug and is typically complete within a week. Drug-dependent antibodies can persist for many years; therefore, it is important that the drug etiology be confirmed and the drug be avoided thereafter.
Conflict of interest statement
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Off-label-drug use: None disclosed.
Figures
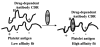
References
-
- George JN, Raskob GE, Shah SR, et al. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Int Med. 1998;129:886–890. - PubMed
-
- Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580–587. - PubMed
-
- Arnold J, Ouwehand WH, Smith G, Cohen H. A young woman with petechiae. Lancet. 1998;352:618. - PubMed
-
- Azuno Y, Yaga K, Sasayama T, Kimoto K. Thrombocytopenia induced by Jui, a traditional Chinese herbal medicine. Lancet. 1999;354:304–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

