Inhibition of soluble epoxide hydrolase preserves cardiomyocytes: role of STAT3 signaling
- PMID: 20008276
- PMCID: PMC2822583
- DOI: 10.1152/ajpheart.00533.2009
Inhibition of soluble epoxide hydrolase preserves cardiomyocytes: role of STAT3 signaling
Abstract
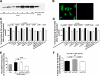
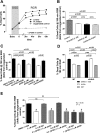
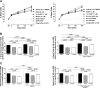
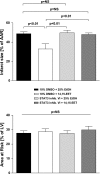
Soluble epoxide hydrolase (sEH) metabolizes epoxyeicosatrienoic acids (EETs), primarily 14,15-EET. EETs are derived from arachidonic acid via P-450 epoxygenases and are cardioprotective. We tested the hypothesis that sEH deficiency and pharmacological inhibition elicit tolerance to ischemia via EET-mediated STAT3 signaling in vitro and in vivo. In addition, the relevance of single nucleotide polymorphisms (SNPs) of EPHX2 (the gene encoding sEH) on tolerance to oxygen and glucose deprivation and reoxygenation and glucose repletion (OGD/RGR) was assessed in male C57BL\6J (WT) or sEH knockout (sEHKO) cardiomyocytes by using transactivator of transcription (TAT)-mediated transduction with sEH mutant proteins. Cell death and hydrolase activity was lower in Arg287Gln EPHX2 mutants vs. nontransduced controls. Excess 14,15-EET and SEH inhibition did not improve cell survival in Arg287Gln mutants. In WT cells, the putative EET receptor antagonist, 14,15-EEZE, abolished the effect of 14,15-EET and sEH inhibition. Cotreatment with 14,15-EET and SEH inhibition did not provide increased protection. In vitro, STAT3 inhibition blocked 14,15-EET cytoprotection, but not the effect of SEH inhibition. However, STAT3 small interfering RNA (siRNA) abolished cytoprotection by 14,15-EET and sEH inhibition, but cells pretreated with JAK2 siRNA remained protected. In vivo, STAT3 inhibition abolished 14,15-EET-mediated infarct size reduction. In summary, the Arg287Gln mutation is associated with improved tolerance against ischemia in vitro, and inhibition of sEH preserves cardiomyocyte viability following OGD/RGR via an EET-dependent mechanism. In vivo and in vitro, 14,15-EET-mediated protection is mediated in part by STAT3.
Figures

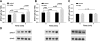

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

