CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions
- PMID: 20008293
- PMCID: PMC2896014
- DOI: 10.4049/jimmunol.0902773
CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions
Abstract
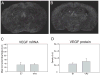
Dysregulation of the blood-brain barrier (BBB) is a hallmark feature of numerous neurologic disorders as diverse as multiple sclerosis, stroke, epilepsy, viral hemorrhagic fevers, cerebral malaria, and acute hemorrhagic leukoencephalitis. CD8 T cells are one immune cell type that have been implicated in promoting vascular permeability in these conditions. Our laboratory has created a murine model of CD8 T cell-mediated CNS vascular permeability using a variation of the Theiler's murine encephalomyelitis virus system traditionally used to study multiple sclerosis. Previously, we demonstrated that CD8 T cells have the capacity to initiate astrocyte activation, cerebral endothelial cell tight junction protein alterations and CNS vascular permeability through a perforin-dependent process. To address the downstream mechanism by which CD8 T cells promote BBB dysregulation, in this study, we assess the role of vascular endothelial growth factor (VEGF) expression in this model. We demonstrate that neuronal expression of VEGF is significantly upregulated prior to, and coinciding with, CNS vascular permeability. Phosphorylation of fetal liver kinase-1 is significantly increased early in this process indicating activation of this receptor. Specific inhibition of neuropilin-1 significantly reduced CNS vascular permeability and fetal liver kinase-1 activation, and preserved levels of the cerebral endothelial cell tight junction protein occludin. Our data demonstrate that CD8 T cells initiate neuronal expression of VEGF in the CNS under neuroinflammatory conditions, and that VEGF may be a viable therapeutic target in neurologic disease characterized by inflammation-induced BBB disruption.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
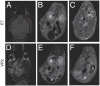

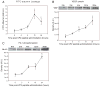
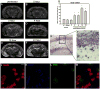
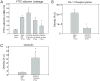
References
-
- Medana IM, Turner GD. Human cerebral malaria and the blood-brain barrier. Int J Parasitol. 2006;36:555–568. - PubMed
-
- Suidan GL, Pirko I, Johnson AJ. A potential role for CD8+ T-cells as regulators of CNS vascular permeability. Neurol Res. 2006;28:250–255. - PubMed
-
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. - PubMed
-
- Kabakus N, Gurgoze MK, Yildirim H, Godekmerdan A, Aydin M. Acute hemorrhagic leukoencephalitis manifesting as intracerebral hemorrhage associated with herpes simplex virus type I. J Trop Pediatr. 2005;51:245–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

