Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis
- PMID: 20008298
- PMCID: PMC4081385
- DOI: 10.1182/blood-2009-10-246363
Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis
Abstract
Few treatment options exist for patients with myelofibrosis (MF), and their survival is significantly shortened. Activating mutation of the JAK2 tyrosine kinase (JAK2(V617F)) is found in approximately 50% of MF patients. CEP-701 is a tyrosine kinase inhibitor that inhibits JAK2 in in vitro and in vivo experiments. We conducted a phase 2 clinical study of CEP-701 in 22 JAK2(V617F)-positive MF patients (80 mg orally twice daily), and 6 (27%) responded by International Working Group criteria (clinical improvement in all cases): reduction in spleen size only (n = 3), transfusion independency (n = 2), and reduction in spleen size with improvement in cytopenias (n = 1). Median time to response was 3 months, and duration of response was more than or equal to 14 months. No improvement was seen in bone marrow fibrosis or JAK2(V617F) allele burden. Phosphorylated STAT3 levels decreased from baseline in responders while on therapy. Eight patients (36%) experienced grade 3 or 4 toxicity, and 6 (27%) required dose reduction. Main side effects were myelosuppression (grade 3 or 4 anemia, 14%; and thrombocytopenia, 23%) and gastrointestinal disturbances (diarrhea, any grade, 72%; grade 3 or 4, 9%; nausea, grade 1 or 2 only, 50%; vomiting, grade 1 or 2 only, 27%). In conclusion, CEP-701 resulted in modest efficacy and mild but frequent gastrointestinal toxicity in MF patients. The study was registered at http://clinicaltrials.gov as NCT00494585.
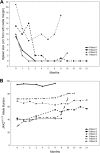
Figures

References
-
- Tefferi A, Gilliland G. Classification of chronic myeloid disorders: from Dameshek towards a semi-molecular system. Best Pract Res Clin Haematol. 2006;19(3):365–385. - PubMed
-
- Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110(4):1092–1097. - PubMed
-
- Mesa RA, Barosi G, Cervantes F, Reilly JT, Tefferi A. Myelofibrosis with myeloid metaplasia: disease overview and non-transplant treatment options. Best Pract Res Clin Haematol. 2006;19(3):495–517. - PubMed
-
- Barosi G, Mesa RA, Thiele J, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22(2):437–438. - PubMed
-
- Dingli D, Mesa RA, Tefferi A. Myelofibrosis with myeloid metaplasia: new developments in pathogenesis and treatment. Intern Med. 2004;43(7):540–547. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

