Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients
- PMID: 20008302
- PMCID: PMC2826759
- DOI: 10.1182/blood-2009-08-239046
Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients
Abstract
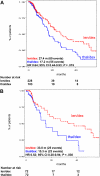
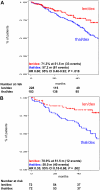
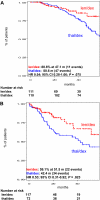
The objective of this case-control study was to compare the efficacy and toxicity of lenalidomide plus dexamethasone (len/dex) versus thalidomide plus dexamethasone (thal/dex) as initial therapy for newly diagnosed myeloma. We retrospectively studied 411 newly diagnosed patients treated with len/dex (228) or thal/dex (183) at the Mayo Clinic. The differences were similar in a matched-pair analysis that adjusted for age, sex, transplantation status, and dexamethasone dose. The proportions of patients achieving at least a partial response to len/dex and thal/dex were 80.3% versus 61.2%, respectively (P < .001); very good partial response rates were 34.2% and 12.0%, respectively (P < .001). Patients receiving len/dex had longer time to progression (median, 27.4 vs 17.2 months; P = .019), progression-free survival (median, 26.7 vs 17.1 months; P = .036), and overall survival (median not reached vs 57.2 months; P = .018). A similar proportion of patients in the 2 groups experienced at least one grade 3 or 4 adverse event (57.5% vs 54.6%, P = .568). Main grade 3 or 4 toxicities of len/dex were hematologic, mainly neutropenia (14.6% vs 0.6%, P < .001); the most common toxicities in thal/dex were venous thromboembolism (15.3% vs 9.2%, P = .058) and peripheral neuropathy (10.4% vs 0.9%, P < .001). Len/dex appears well-tolerated and more effective than thal/dex. Randomized trials are needed to confirm these results.
Figures

Comment in
-
Lenalidomide for multiple myeloma.Nat Rev Clin Oncol. 2010 May;7(5):241. doi: 10.1038/nrclinonc.2010.55. Nat Rev Clin Oncol. 2010. PMID: 20432526 No abstract available.
References
-
- Rajkumar SV, Kyle RA. Plasma cell disorders. In: Goldman L, Ausiello D, editors. Cecil Textbook of Medicine. 23d ed. Philadelphia, PA: Saunders; 2008. pp. 1426–1437.
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. - PubMed
-
- Alexanian R, Barlogie B, Tucker S. VAD-based regimens as primary treatment for multiple myeloma. Am J Hematol. 1990;33:86–89. - PubMed
-
- Sirohi B, Powles R. Multiple myeloma. Lancet. 2004;363:875–887. - PubMed
-
- Rajkumar SV, Blood E, Vesole DH, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

