IIp45 inhibits cell migration through inhibition of HDAC6
- PMID: 20008322
- PMCID: PMC2823495
- DOI: 10.1074/jbc.M109.063354
IIp45 inhibits cell migration through inhibition of HDAC6
Abstract
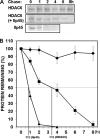
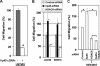
IIp45 (aka MIIP) is a newly discovered gene whose protein product inhibits cell migration. HDAC6 is a class IIb deacetylase that specifically deacetylates alpha-tubulin, modulates microtubule dynamics, and promotes cell migration. A yeast two-hybrid assay using IIp45 as bait identified HDAC6 protein as a binding partner of IIp45. This physical interaction of the two functionally antagonistic proteins was confirmed by glutathione S-transferase pulldown assay and co-immunoprecipitation assay in human cells. Serial deletion constructs of HDAC6 were used to characterize the interaction of HDAC6 and IIp45, and this analysis found that the two catalytic domains of HDAC6 protein are required for IIp45 binding. We examined the protein expression patterns of IIp45 and HDAC6 in glioma tissues. Elevated protein levels of HDAC6 were found in high grade glioma samples, in contrast to the decreased protein expression of IIp45. The potential negative regulation of HDAC6 expression by IIp45 was confirmed in cell lines with altered IIp45 expression by constitutive overexpression or small interfering RNA knockdown. Protein turnover study revealed that overexpression of IIp45 significantly reduces the intracellular protein stability of endogenous HDAC6, indicating a possible mechanism for the negative regulation of HDAC6 by IIp45. Results from the HDAC activity assay demonstrated that overexpressed IIp45 effectively decreases HDAC6 activity, increases acetylated alpha-tubulin, and reduces cell migration. The increased cell migration resulting from siIIp45 knockdown was significantly reversed by co-transfection of siHDAC6. Thus, we report here for the first time a novel mechanism by which IIp45 inhibits cell motility through inhibition of HDAC6.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

