Molecular insights into mammalian end-binding protein heterodimerization
- PMID: 20008324
- PMCID: PMC2820806
- DOI: 10.1074/jbc.M109.068130
Molecular insights into mammalian end-binding protein heterodimerization
Abstract
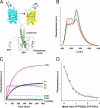
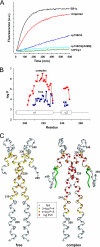
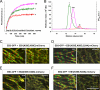
Microtubule plus-end tracking proteins (+TIPs) are involved in many microtubule-based processes. End binding (EB) proteins constitute a highly conserved family of +TIPs. They play a pivotal role in regulating microtubule dynamics and in the recruitment of diverse +TIPs to growing microtubule plus ends. Here we used a combination of methods to investigate the dimerization properties of the three human EB proteins EB1, EB2, and EB3. Based on Förster resonance energy transfer, we demonstrate that the C-terminal dimerization domains of EBs (EBc) can readily exchange their chains in solution. We further document that EB1c and EB3c preferentially form heterodimers, whereas EB2c does not participate significantly in the formation of heterotypic complexes. Measurements of the reaction thermodynamics and kinetics, homology modeling, and mutagenesis provide details of the molecular determinants of homo- versus heterodimer formation of EBc domains. Fluorescence spectroscopy and nuclear magnetic resonance studies in the presence of the cytoskeleton-associated protein-glycine-rich domains of either CLIP-170 or p150(glued) or of a fragment derived from the adenomatous polyposis coli tumor suppressor protein show that chain exchange of EBc domains can be controlled by binding partners. Extension of these studies of the EBc domains to full-length EBs demonstrate that heterodimer formation between EB1 and EB3, but not between EB2 and the other two EBs, occurs both in vitro and in cells as revealed by live cell imaging. Together, our data provide molecular insights for rationalizing the dominant negative control by C-terminal EB domains and form a basis for understanding the functional role of heterotypic chain exchange by EBs in cells.
Figures
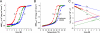

Similar articles
-
Mammalian end binding proteins control persistent microtubule growth.J Cell Biol. 2009 Mar 9;184(5):691-706. doi: 10.1083/jcb.200807179. Epub 2009 Mar 2. J Cell Biol. 2009. PMID: 19255245 Free PMC article.
-
Interaction of mammalian end binding proteins with CAP-Gly domains of CLIP-170 and p150(glued).J Struct Biol. 2012 Jan;177(1):160-7. doi: 10.1016/j.jsb.2011.11.010. Epub 2011 Nov 17. J Struct Biol. 2012. PMID: 22119847
-
EB1 and EB3 control CLIP dissociation from the ends of growing microtubules.Mol Biol Cell. 2005 Nov;16(11):5334-45. doi: 10.1091/mbc.e05-07-0614. Epub 2005 Sep 7. Mol Biol Cell. 2005. PMID: 16148041 Free PMC article.
-
Structural basis of EB1 effects on microtubule dynamics.Biochem Soc Trans. 2009 Oct;37(Pt 5):997-1001. doi: 10.1042/BST0370997. Biochem Soc Trans. 2009. PMID: 19754439 Review.
-
Capturing protein tails by CAP-Gly domains.Trends Biochem Sci. 2008 Nov;33(11):535-45. doi: 10.1016/j.tibs.2008.08.006. Epub 2008 Oct 4. Trends Biochem Sci. 2008. PMID: 18835717 Review.
Cited by
-
Microtubule Plus End Dynamics - Do We Know How Microtubules Grow?: Cells boost microtubule growth by promoting distinct structural transitions at growing microtubule ends.Bioessays. 2019 Mar;41(3):e1800194. doi: 10.1002/bies.201800194. Epub 2019 Feb 7. Bioessays. 2019. PMID: 30730055 Free PMC article.
-
Microtubule plus-end tracking proteins in neuronal development.Cell Mol Life Sci. 2016 May;73(10):2053-77. doi: 10.1007/s00018-016-2168-3. Epub 2016 Mar 11. Cell Mol Life Sci. 2016. PMID: 26969328 Free PMC article. Review.
-
SxIP binding disrupts the constitutive homodimer interface of EB1 and stabilizes EB1 monomer.Biophys J. 2021 May 18;120(10):2019-2029. doi: 10.1016/j.bpj.2021.03.004. Epub 2021 Mar 16. Biophys J. 2021. PMID: 33737159 Free PMC article.
-
The HCMV Assembly Compartment Is a Dynamic Golgi-Derived MTOC that Controls Nuclear Rotation and Virus Spread.Dev Cell. 2018 Apr 9;45(1):83-100.e7. doi: 10.1016/j.devcel.2018.03.010. Dev Cell. 2018. PMID: 29634939 Free PMC article.
-
The Leber congenital amaurosis protein AIPL1 and EB proteins co-localize at the photoreceptor cilium.PLoS One. 2015 Mar 23;10(3):e0121440. doi: 10.1371/journal.pone.0121440. eCollection 2015. PLoS One. 2015. PMID: 25799540 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

