Electrophysiological identification of glucose-sensing neurons in rat nodose ganglia
- PMID: 20008464
- PMCID: PMC2828136
- DOI: 10.1113/jphysiol.2009.182147
Electrophysiological identification of glucose-sensing neurons in rat nodose ganglia
Abstract
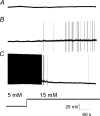
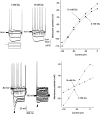
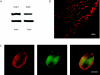
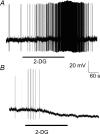
The vagal afferent system is strategically positioned to mediate rapid changes in motility and satiety in response to systemic glucose levels. In the present study we aimed to identify glucose-excited and glucose-inhibited neurons in nodose ganglia and characterize their glucose-sensing properties. Whole-cell patch-clamp recordings in vagal afferent neurons isolated from rat nodose ganglia demonstrated that 31/118 (26%) neurons were depolarized after increasing extracellular glucose from 5 to 15 mm; 19/118 (16%) were hyperpolarized, and 68/118 were non-responsive. A higher incidence of excitatory response to glucose occurred in gastric- than in portal vein-projecting neurons, the latter having a higher incidence of inhibitory response. In glucose-excited neurons, elevated glucose evoked membrane depolarization (11 mV) and an increase in membrane input resistance (361 to 437 M). Current reversed at 99 mV. In glucose-inhibited neurons, membrane hyperpolarization (13 mV) was associated with decreased membrane input resistance (383 to 293 M). Current reversed at 97 mV. Superfusion of tolbutamide, a K(ATP) channel sulfonylurea receptor blocker, elicited identical glucose-excitatory but not glucose-inhibitory responses. Kir6.2 shRNA transfection abolished glucose-excited but not glucose-inhibited responses. Phosphatidylinositol bisphosphate (PIP(2)) depletion using wortmannin increased the fraction of glucose-excited neurons from 26% to 80%. These results show that rat nodose ganglia have glucose-excited and glucose-inhibited neurons, differentially distributed among gastric- and portal vein-projecting nodose neurons. In glucose-excited neurons, glucose metabolism leads to K(ATP) channel closure, triggering membrane depolarization, whereas in glucose-inhibited neurons, the inhibitory effect of elevated glucose is mediated by an ATP-independent K(+) channel. The results also show that PIP(2) can determine the excitability of glucose-excited neurons.
Figures



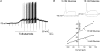
References
-
- Adachi A, Shimizu N, Oomura Y, Kobashi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett. 1984;46:215–218. - PubMed
-
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. - PubMed
-
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions

