Development and initial validation of the localized scleroderma skin damage index and physician global assessment of disease damage: a proof-of-concept study
- PMID: 20008472
- PMCID: PMC3498950
- DOI: 10.1093/rheumatology/kep361
Development and initial validation of the localized scleroderma skin damage index and physician global assessment of disease damage: a proof-of-concept study
Abstract
Objective: To develop and assess the psychometric properties of the Localized Scleroderma (LS) Skin Damage Index (LoSDI) and Physician Global Assessment of disease Damage (PGA-D).
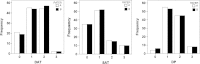
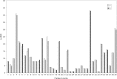
Methods: Damage was defined as irreversible/persistent changes (>6 months) due to previous active disease/complications of therapy. Eight rheumatologists assessed the importance of 17 variables in formulating the PGA-D/LoSDI. LS patients were evaluated by two rheumatologists using both tools to assess their psychometric properties. LoSDI was calculated by summing three scores for cutaneous features of damage [dermal atrophy (DAT), subcutaneous atrophy (SAT) and dyspigmentation (DP)] measured at 18 anatomic sites. Patient GA of disease severity (PtGA-S), Children's Dermatology Life Quality Index (CDLQI) and PGA-D were recorded at the time of each examination.
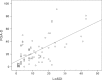
Results: Thirty LS patients (112 lesions) and nine patient-visit pairs (18 lesions) were included for inter- and intra-rater reliability study. LoSDI and its domains DAT, SAT, DP and PGA-D demonstrated excellent inter- and intra-rater reliability (reliability coefficients 0.86-0.99 and 0.74-0.96, respectively). LoSDI correlated moderately with PGA-D and poorly with PtGA-S and CDLQI. PGA-D correlated moderately with PtGA-S, but poorly with CDLQI.
Conclusions: To complete the LS Cutaneous Assessment Tool (LoSCAT), we developed and evaluated the psychometric properties of the LoSDI and PGA-D in addition to the LS Skin Severity Index (LoSSI). These instruments will facilitate evaluation of LS patients for individual patient management and clinical trials. LoSDI and PGA-D demonstrated excellent reliability and high validity. LoSCAT provides an improved understanding of LS natural history. Further study in a larger group of patients is needed to confirm these preliminary findings.
Figures

References
-
- Torres JE, Sanchez JL. Histopathologic differentiation between localized and systemic scleroderma. Am J Dermatopathol. 1998;20:242–5. - PubMed
-
- Peterson LS, Nelson AM, Su WP, Mason T, O’Fallon WM, Gabriel SE. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960–1993. J Rheumatol. 1997;24:73–80. - PubMed
-
- Arkachaisri T, Pino S. Localized scleroderma severity index and global assessments: a pilot study of outcome instruments. J Rheumatol. 2008;35:650–7. - PubMed
-
- Marzano AV, Menni S, Parodi A, et al. Localized scleroderma in adults and children. Clinical and laboratory investigations on 239 cases. Eur J Dermatol. 2003;13:171–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

