New aspects of the interaction of the antibiotic coralyne with RNA: coralyne induces triple helix formation in poly(rA)*poly(rU)
- PMID: 20008509
- PMCID: PMC2836573
- DOI: 10.1093/nar/gkp1146
New aspects of the interaction of the antibiotic coralyne with RNA: coralyne induces triple helix formation in poly(rA)*poly(rU)
Abstract
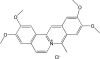
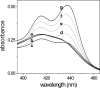
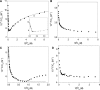
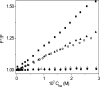
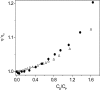
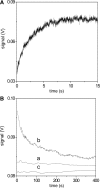
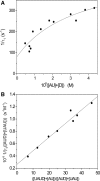
The interaction of coralyne with poly(A)*poly(U), poly(A)*2poly(U), poly(A) and poly(A)*poly(A) is analysed using spectrophotometric, spectrofluorometric, circular dichroism (CD), viscometric, stopped-flow and temperature-jump techniques. It is shown for the first time that coralyne induces disproportionation of poly(A)*poly(U) to triplex poly(A)*2poly(U) and single-stranded poly(A) under suitable values of the [dye]/[polymer] ratio (C(D)/C(P)). Kinetic, CD and spectrofluorometric experiments reveal that this process requires that coralyne (D) binds to duplex. The resulting complex (AUD) reacts with free duplex giving triplex (UAUD) and free poly(A); moreover, ligand exchange between duplex and triplex occurs. A reaction mechanism is proposed and the reaction parameters are evaluated. For C(D)/C(P)> 0.8 poly(A)*poly(U) does not disproportionate at 25 degrees C and dye intercalation into AU to give AUD is the only observed process. Melting experiments as well show that coralyne induces the duplex disproportionation. Effects of temperature, ionic strength and ethanol content are investigated. One concludes that triplex formation requires coralyne be only partially intercalated into AUD. Under suitable concentration conditions, this feature favours the interaction of free AU with AUD to give the AUDAU intermediate which evolves into triplex UAUD and single-stranded poly(A). Duplex poly(A)*poly(A) undergoes aggregation as well, but only at much higher polymer concentrations compared to poly(A)*poly(U).
Figures


Similar articles
-
RNA triplex-to-duplex and duplex-to-triplex conversion induced by coralyne.Phys Chem Chem Phys. 2014 Apr 7;16(13):6012-8. doi: 10.1039/c3cp52270a. Phys Chem Chem Phys. 2014. PMID: 24553832
-
Complete disproportionation of duplex poly(dT)*poly(dA) into triplex poly(dT)*poly(dA)*poly(dT) and poly(dA) by coralyne.Nucleic Acids Res. 2002 Feb 15;30(4):983-92. doi: 10.1093/nar/30.4.983. Nucleic Acids Res. 2002. PMID: 11842110 Free PMC article.
-
Mg(II) and Ni(II) induce aggregation of poly(rA)poly(rU) to either tetra-aggregate or triplex depending on the metal ion concentration.J Inorg Biochem. 2015 Oct;151:115-22. doi: 10.1016/j.jinorgbio.2015.05.001. Epub 2015 May 9. J Inorg Biochem. 2015. PMID: 26004214
-
Controlling nucleic acid secondary structure by intercalation: effects of DNA strand length on coralyne-driven duplex disproportionation.Nucleic Acids Res. 2003 Aug 1;31(15):4608-15. doi: 10.1093/nar/gkg648. Nucleic Acids Res. 2003. PMID: 12888521 Free PMC article.
-
Oligo-Adenine Derived Secondary Nucleic Acid Frameworks: From Structural Characteristics to Applications.Angew Chem Int Ed Engl. 2024 Nov 4;63(45):e202412106. doi: 10.1002/anie.202412106. Epub 2024 Oct 4. Angew Chem Int Ed Engl. 2024. PMID: 39183707 Review.
Cited by
-
Natural and artificial binders of polyriboadenylic acid and their effect on RNA structure.Beilstein J Nanotechnol. 2015 Jun 17;6:1338-47. doi: 10.3762/bjnano.6.138. eCollection 2015. Beilstein J Nanotechnol. 2015. PMID: 26199837 Free PMC article. Review.
-
RNA targeting by small molecules: Binding of protoberberine, benzophenanthridine and aristolochia alkaloids to various RNA structures.J Biosci. 2012 Jul;37(3):539-52. doi: 10.1007/s12038-012-9217-3. J Biosci. 2012. PMID: 22750990 Review.
-
Discovery of Small Molecule Ligands for MALAT1 by Tuning an RNA-Binding Scaffold.Angew Chem Int Ed Engl. 2018 Oct 1;57(40):13242-13247. doi: 10.1002/anie.201808823. Epub 2018 Sep 10. Angew Chem Int Ed Engl. 2018. PMID: 30134013 Free PMC article.
-
Three- and four-stranded nucleic acid structures and their ligands.RSC Chem Biol. 2025 Feb 19;6(4):466-491. doi: 10.1039/d4cb00287c. eCollection 2025 Apr 2. RSC Chem Biol. 2025. PMID: 40007865 Free PMC article. Review.
-
Unexpected complex formation between coralyne and cyclic diadenosine monophosphate providing a simple fluorescent turn-on assay to detect this bacterial second messenger.Anal Chem. 2014 Mar 4;86(5):2412-20. doi: 10.1021/ac403203x. Epub 2014 Feb 19. Anal Chem. 2014. PMID: 24494631 Free PMC article.
References
-
- Gatto B, Sanders MM, Yu C, Wu HY, Mahkey D, LaVoie EJ, Liu LF. Identification of Topoisomerase I as the cytotoxic target of the protoberberine alkaloid coralyne. Cancer Res. 1996;56:2795–2800. - PubMed
-
- Zee-Cheng K, Paull KD, Cheng CC. Experimental antileukemic agents. Coralyne, analogs, and related compounds. J. Med. Chem. 1974;17:347–351. - PubMed
-
- Whang L, Rogers BD, Hecht SM. Inhibition of Topoisomerase I function by coralyne and 5,6-dihydrocoralyne. Chem. Res. Toxicol. 1996;9:75–83. - PubMed
-
- Maiti M, Kumar GS. Protoberberine alkaloids. Physicochemical and nucleic acid binding properties. Top. Heterocyclic Chem. 2007;10:155–209.
-
- Maiti M, Kumar GS. Molecular aspects on the interaction of protoberberine, benzophenanthridine, and aristolochia group of alkaloids with nucleic acid structures and biological perspectives. Med. Res. Rev. 2007;27:649–695. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

