The origin and development of nonlymphoid tissue CD103+ DCs
- PMID: 20008528
- PMCID: PMC2806447
- DOI: 10.1084/jem.20091756
The origin and development of nonlymphoid tissue CD103+ DCs
Abstract
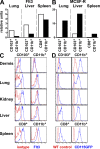
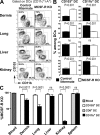
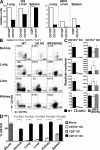
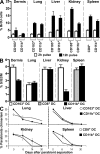
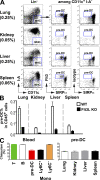
CD103(+) dendritic cells (DCs) in nonlymphoid tissues are specialized in the cross-presentation of cell-associated antigens. However, little is known about the mechanisms that regulate the development of these cells. We show that two populations of CD11c(+)MHCII(+) cells separated on the basis of CD103 and CD11b expression coexist in most nonlymphoid tissues with the exception of the lamina propria. CD103(+) DCs are related to lymphoid organ CD8(+) DCs in that they are derived exclusively from pre-DCs under the control of fms-like tyrosine kinase 3 (Flt3) ligand, inhibitor of DNA protein 2 (Id2), and IFN regulatory protein 8 (IRF8). In contrast, lamina propria CD103(+) DCs express CD11b and develop independently of Id2 and IRF8. The other population of CD11c(+)MHCII(+) cells in tissues, which is CD103(-)CD11b(+), is heterogenous and depends on both Flt3 and MCSF-R. Our results reveal that nonlymphoid tissue CD103(+) DCs and lymphoid organ CD8(+) DCs derive from the same precursor and follow a related differentiation program.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

