Toll-like receptor 4 signaling leads to severe fungal infection associated with enhanced proinflammatory immunity and impaired expansion of regulatory T cells
- PMID: 20008536
- PMCID: PMC2825906
- DOI: 10.1128/IAI.01198-09
Toll-like receptor 4 signaling leads to severe fungal infection associated with enhanced proinflammatory immunity and impaired expansion of regulatory T cells
Abstract
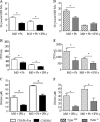
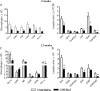
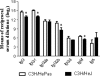
Toll-like receptors (TLRs) present in innate immune cells recognize pathogen molecular patterns and influence immunity to control the host-parasite interaction. The objective of this study was to characterize the involvement of TLR4 in the innate and adaptive immunity to Paracoccidioides brasiliensis, the most important primary fungal pathogen of Latin America. We compared the responses of C3H/HeJ mice, which are naturally defective in TLR4 signaling, with those of C3H/HePas mice, which express functional receptors, after in vitro and in vivo infection with P. brasiliensis. Unexpectedly, we verified that TLR4-defective macrophages infected in vitro with P. brasiliensis presented decreased fungal loads associated with impaired synthesis of nitric oxide, interleukin-12 (IL-12), and macrophage chemotactic protein 1 (MCP-1). After intratracheal infection with 1 million yeasts, TLR4-defective mice developed reduced fungal burdens and decreased levels of pulmonary nitric oxide, proinflammatory cytokines, and antibodies. TLR4-competent mice produced elevated levels of IL-12 and tumor necrosis factor alpha (TNF-alpha), besides cytokines of the Th17 pattern, indicating a proinflammatory role for TLR4 signaling. The more severe infection of TLR4-normal mice resulted in increased influx of activated macrophages and T cells to the lungs and progressive control of fungal burdens but impaired expansion of regulatory T cells (Treg cells). In contrast, TLR4-defective mice were not able to clear their diminished fungal burdens totally, a defect associated with deficient activation of T-cell immunity and enhanced development of Treg cells. These divergent patterns of immunity, however, resulted in equivalent mortality rates, indicating that control of elevated fungal growth mediated by vigorous inflammatory reactions is as deleterious to the hosts as low fungal loads inefficiently controlled by limited inflammatory reactions.
Figures





Similar articles
-
TNF-α and CD8+ T cells mediate the beneficial effects of nitric oxide synthase-2 deficiency in pulmonary paracoccidioidomycosis.PLoS Negl Trop Dis. 2013 Aug 1;7(8):e2325. doi: 10.1371/journal.pntd.0002325. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23936574 Free PMC article.
-
In pulmonary paracoccidioidomycosis IL-10 deficiency leads to increased immunity and regressive infection without enhancing tissue pathology.PLoS Negl Trop Dis. 2013 Oct 24;7(10):e2512. doi: 10.1371/journal.pntd.0002512. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24205424 Free PMC article.
-
MyD88 signaling is required for efficient innate and adaptive immune responses to Paracoccidioides brasiliensis infection.Infect Immun. 2011 Jun;79(6):2470-80. doi: 10.1128/IAI.00375-10. Epub 2011 Mar 21. Infect Immun. 2011. PMID: 21422180 Free PMC article.
-
Toll-like receptors and fungal infections: the role of TLR2, TLR4 and MyD88 in paracoccidioidomycosis.FEMS Immunol Med Microbiol. 2008 Jun;53(1):1-7. doi: 10.1111/j.1574-695X.2008.00378.x. Epub 2008 Apr 1. FEMS Immunol Med Microbiol. 2008. PMID: 18384366 Review.
-
Regulatory T cells in paracoccidioidomycosis.Virulence. 2019 Dec;10(1):810-821. doi: 10.1080/21505594.2018.1483674. Epub 2018 Aug 1. Virulence. 2019. PMID: 30067137 Free PMC article. Review.
Cited by
-
CD28 exerts protective and detrimental effects in a pulmonary model of paracoccidioidomycosis.Infect Immun. 2010 Nov;78(11):4922-35. doi: 10.1128/IAI.00297-10. Epub 2010 Aug 16. Infect Immun. 2010. PMID: 20713624 Free PMC article.
-
Myeloid-derived suppressor cells are associated with impaired Th1 and Th17 responses and severe pulmonary paracoccidioidomycosis which is reversed by anti-Gr1 therapy.Front Immunol. 2023 Jan 26;14:1039244. doi: 10.3389/fimmu.2023.1039244. eCollection 2023. Front Immunol. 2023. PMID: 36776848 Free PMC article.
-
TLR3 Is a Negative Regulator of Immune Responses Against Paracoccidioides brasiliensis.Front Cell Infect Microbiol. 2019 Jan 10;8:426. doi: 10.3389/fcimb.2018.00426. eCollection 2018. Front Cell Infect Microbiol. 2019. PMID: 30687643 Free PMC article.
-
The immunosuppressive activity of myeloid-derived suppressor cells in murine Paracoccidioidomycosis relies on Indoleamine 2,3-dioxygenase activity and Dectin-1 and TLRs signaling.Sci Rep. 2023 Jul 31;13(1):12391. doi: 10.1038/s41598-023-39262-8. Sci Rep. 2023. PMID: 37524886 Free PMC article.
-
NOD-Like Receptor P3 Inflammasome Controls Protective Th1/Th17 Immunity against Pulmonary Paracoccidioidomycosis.Front Immunol. 2017 Jul 10;8:786. doi: 10.3389/fimmu.2017.00786. eCollection 2017. Front Immunol. 2017. PMID: 28740491 Free PMC article.
References
-
- Abdollahi-Roodsaz, S., L. A. Joosten, M. I. Koenders, I. Devesa, M. F. Roelofs, T. R. Radstake, M. Heuvelmans-Jacobs, S. Akira, M. J. Nicklin, F. Ribeiro-Dias, and W. B. van den Berg. 2008. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118:205-216. - PMC - PubMed
-
- Akira, A. 2006. TLR signaling. Curr. Top. Microbiol. Immunol. 311:1-16. - PubMed
-
- Anand, R. J., J. W. Kohler, J. A. Cavallo, J. Li, T. Dubowski, and D. J. Hackam. 2007. Toll-like receptor 4 plays a role in macrophage phagocytosis during peritoneal sepsis. J. Pediatr. Surg. 42:927-932. - PubMed
-
- Belkaid, Y., and B. T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353-360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

