Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells
- PMID: 20008545
- PMCID: PMC2879945
- DOI: 10.1096/fj.09-147025
Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells
Abstract
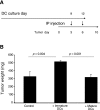
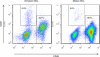
Dendritic cells (DCs)--immunomodulatory cells that initiate adaptive immune responses--have recently been shown to exert proangiogenic effects when infiltrating the tumor microenvironment. As tumors that escape immune surveillance inhibit DC maturation, we explored whether maturation status determines their ability to promote angiogenesis and whether angiogenesis depends on the presence of DCs. Using mouse xenograft models of human tumors, we show that fast-growing "angiogenic" tumors are infiltrated by a more immature DC population than respective dormant avascular tumors. Accordingly, supplementation of immature DCs, but not mature DCs, enhanced tumor growth. When DCs were mixed with Matrigel and injected subcutaneously into mice, only immature DCs promoted the ingrowth of patent blood vessels. Notably, depletion of DCs in a transgenic mouse model that allows for their conditional ablation completely abrogated basic fibroblast growth factor-induced angiogenesis in Matrigel plugs, and significantly inhibited tumor growth in these mice. Because immature DCs actively promote angiogenesis and tumor growth, whereas DC maturation or ablation suppresses this response, we conclude that angiogenesis is dependent on the presence of immature DCs. Thus, cancer immunotherapies that promote DC maturation may act by both augmenting the host immune response to the tumor and by suppressing tumor angiogenesis.
Figures



Similar articles
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
-
Dendritic cells and their role in tumor immunosurveillance.Innate Immun. 2013 Feb;19(1):98-111. doi: 10.1177/1753425912449549. Epub 2012 Jun 25. Innate Immun. 2013. PMID: 22732734 Review.
-
In vivo manipulation of dendritic cell migration and activation to elicit antitumour immunity.Novartis Found Symp. 2004;256:241-54; discussion 254-69. doi: 10.1002/0470856734.ch18. Novartis Found Symp. 2004. PMID: 15027495 Review.
-
Targeting adrenomedullin receptors with systemic delivery of neutralizing antibodies inhibits tumor angiogenesis and suppresses growth of human tumor xenografts in mice.FASEB J. 2009 Oct;23(10):3424-35. doi: 10.1096/fj.08-127852. Epub 2009 Jun 22. FASEB J. 2009. PMID: 19546305
-
Protective anti-tumour immune responses by murine dendritic cells pulsed with recombinant Tat-carcinoembryonic antigen derived from Escherichia coli.Clin Exp Immunol. 2009 Jul;157(1):128-38. doi: 10.1111/j.1365-2249.2009.03943.x. Clin Exp Immunol. 2009. PMID: 19659778 Free PMC article.
Cited by
-
Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients.BMC Cancer. 2015 Mar 28;15:195. doi: 10.1186/s12885-015-1204-2. BMC Cancer. 2015. PMID: 25884918 Free PMC article.
-
An immunocompetent mouse model of human glioblastoma.Oncotarget. 2017 May 15;8(37):61072-61082. doi: 10.18632/oncotarget.17851. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 28977847 Free PMC article.
-
Manipulation of the crosstalk between tumor angiogenesis and immunosuppression in the tumor microenvironment: Insight into the combination therapy of anti-angiogenesis and immune checkpoint blockade.Front Immunol. 2022 Nov 10;13:1035323. doi: 10.3389/fimmu.2022.1035323. eCollection 2022. Front Immunol. 2022. PMID: 36439137 Free PMC article. Review.
-
Melanoma-infiltrating dendritic cells: Limitations and opportunities of mouse models.Oncoimmunology. 2012 Dec 1;1(9):1584-1593. doi: 10.4161/onci.22660. Oncoimmunology. 2012. PMID: 23264904 Free PMC article.
-
Caveolin-1 promotes glioma proliferation and metastasis by enhancing EMT via mediating PAI-1 activation and its correlation with immune infiltrates.Heliyon. 2024 Jan 14;10(2):e24464. doi: 10.1016/j.heliyon.2024.e24464. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38298655 Free PMC article.
References
-
- Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Steinman R M, Hawiger D, Nussenzweig M C. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. - PubMed
-
- Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. - PubMed
-
- Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

