The Caenorhabditis elegans Ste20-related kinase and Rac-type small GTPase regulate the c-Jun N-terminal kinase signaling pathway mediating the stress response
- PMID: 20008556
- PMCID: PMC2815562
- DOI: 10.1128/MCB.01131-09
The Caenorhabditis elegans Ste20-related kinase and Rac-type small GTPase regulate the c-Jun N-terminal kinase signaling pathway mediating the stress response
Abstract
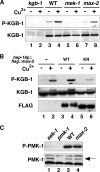
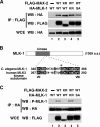
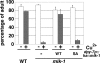
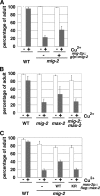
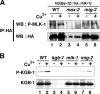
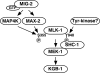
Mitogen-activated protein kinases (MAPKs) are integral to the mechanisms by which cells respond to physiological stimuli and a wide variety of environmental stresses. In Caenorhabditis elegans, the stress response is controlled by a c-Jun N-terminal kinase (JNK)-like MAPK signaling pathway, which is regulated by MLK-1 MAPK kinase kinase (MAPKKK), MEK-1 MAPKK, and KGB-1 JNK-like MAPK. In this study, we identify the max-2 gene encoding a C. elegans Ste20-related protein kinase as a component functioning upstream of the MLK-1-MEK-1-KGB-1 pathway. The max-2 loss-of-function mutation is defective in activation of KGB-1, resulting in hypersensitivity to heavy metals. Biochemical analysis reveals that MAX-2 activates MLK-1 through direct phosphorylation of a specific residue in the activation loop of the MLK-1 kinase domain. Our genetic data presented here also show that MIG-2 small GTPase functions upstream of MAX-2 in the KGB-1 pathway. These results suggest that MAX-2 and MIG-2 play a crucial role in mediating the heavy metal stress response regulated by the KGB-1 pathway.
Figures



References
-
- Bossemeyer, D. 1995. Protein kinases—structure and function. FEBS Lett. 369:57-61. - PubMed
-
- Byrd, D. T., M. Kawasaki, M. Walcoff, N. Hisamoto, K. Matsumoto, and Y. Jin. 2001. UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron 32:787-800. - PubMed
-
- Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. - PubMed
-
- Dan, I., N. M. Watanabe, and A. Kusumi. 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell. Biol. 11:220-230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
