Basal body stability and ciliogenesis requires the conserved component Poc1
- PMID: 20008567
- PMCID: PMC2806327
- DOI: 10.1083/jcb.200908019
Basal body stability and ciliogenesis requires the conserved component Poc1
Abstract
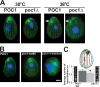
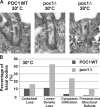
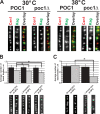
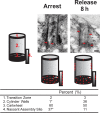
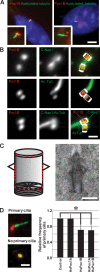
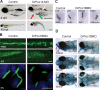
Centrioles are the foundation for centrosome and cilia formation. The biogenesis of centrioles is initiated by an assembly mechanism that first synthesizes the ninefold symmetrical cartwheel and subsequently leads to a stable cylindrical microtubule scaffold that is capable of withstanding microtubule-based forces generated by centrosomes and cilia. We report that the conserved WD40 repeat domain-containing cartwheel protein Poc1 is required for the structural maintenance of centrioles in Tetrahymena thermophila. Furthermore, human Poc1B is required for primary ciliogenesis, and in zebrafish, DrPoc1B knockdown causes ciliary defects and morphological phenotypes consistent with human ciliopathies. T. thermophila Poc1 exhibits a protein incorporation profile commonly associated with structural centriole components in which the majority of Poc1 is stably incorporated during new centriole assembly. A second dynamic population assembles throughout the cell cycle. Our experiments identify novel roles for Poc1 in centriole stability and ciliogenesis.
Figures

References
-
- Azimzadeh J., Bornens M.. 2004. The centrosome in evolution. In Centrosomes in Development and Disease. Nigg E.A., editor. Wiley-VCH, Weinheim, Germany. 431 pp.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

