The chromatin-remodeling protein Osa interacts with CyclinE in Drosophila eye imaginal discs
- PMID: 20008573
- PMCID: PMC2845341
- DOI: 10.1534/genetics.109.109967
The chromatin-remodeling protein Osa interacts with CyclinE in Drosophila eye imaginal discs
Abstract
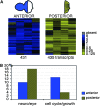
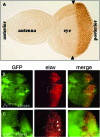
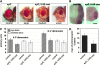
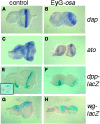
Coordinating cell proliferation and differentiation is essential during organogenesis. In Drosophila, the photoreceptor, pigment, and support cells of the eye are specified in an orchestrated wave as the morphogenetic furrow passes across the eye imaginal disc. Cells anterior of the furrow are not yet differentiated and remain mitotically active, while most cells in the furrow arrest at G(1) and adopt specific ommatidial fates. We used microarray expression analysis to monitor changes in transcription at the furrow and identified genes whose expression correlates with either proliferation or fate specification. Some of these are members of the Polycomb and Trithorax families that encode epigenetic regulators. Osa is one; it associates with components of the Drosophila SWI/SNF chromatin-remodeling complex. Our studies of this Trithorax factor in eye development implicate Osa as a regulator of the cell cycle: Osa overexpression caused a small-eye phenotype, a reduced number of M- and S-phase cells in eye imaginal discs, and a delay in morphogenetic furrow progression. In addition, we present evidence that Osa interacts genetically and biochemically with CyclinE. Our results suggest a dual mechanism of Osa function in transcriptional regulation and cell cycle control.
Figures



References
-
- Alvarez-Venegas, R., and Z. Avramova, 2002. SET-domain proteins of the Su(var)3–9, E(z) and trithorax families. Gene 285 25–37. - PubMed
-
- Baker, N. E., 2001. Cell proliferation, survival, and death in the Drosophila eye. Semin. Cell Dev. Biol. 12 499–507. - PubMed
-
- Baker, N. E., 2007. Patterning signals and proliferation in Drosophila imaginal discs. Curr. Opin. Genet. Dev. 17 287–293. - PubMed
-
- Baker, N. E., and S. Y. Yu, 1997. Proneural function of neurogenic genes in the developing Drosophila eye. Curr. Biol. 7 122–132. - PubMed
-
- Baonza, A., and M. Freeman, 2002. Control of Drosophila eye specification by Wingless signalling. Development 129 5313–5322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

