Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders
- PMID: 20008633
- PMCID: PMC2815704
- DOI: 10.1200/JCO.2009.24.1570
Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders
Abstract
Purpose: Flavopiridol downmodulates antiapoptotic proteins associated with resistance to fludarabine and rituximab and is effective against p53-mutated chronic lymphocytic leukemia (CLL). We conducted a phase I study of flavopiridol, fludarabine, and rituximab (FFR) in patients with mantle-cell lymphoma (MCL), indolent B-cell non-Hodgkin's lymphomas (B-NHL), and CLL to determine the activity of FFR.
Patients and methods: Therapy included fludarabine 25 mg/m(2) intravenously (IV) days 1 to 5 and rituximab 375 mg/m(2) day 1 every 28 days for 6 cycles. We administered flavopiridol 50 mg/m(2) by 1-hour IV bolus (IVB) day 1 (n = 15); day 1 to 2 (n = 6); 20 mg/m(2) 30-minute IVB + 20 mg/m(2) 4-hour IV infusion (n = 3); or 30 mg/m(2) + 30 mg/m(2) (n = 14).
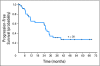
Results: Thirty-eight patients (median age, 62 years) with MCL (n = 10); indolent B-NHL including follicular (n = 9), marginal zone (n = 4), lymphoplasmacytic (n = 1), or small lymphocytic lymphoma (n = 3); and CLL (n = 11), were enrolled. Twenty-two patients were previously untreated; 16 had received one to two prior therapies. Two patients in cohort 2 developed grade 3 dose-limiting toxicity (seizures, renal insufficiency). The median number of treatment cycles was 4, with cytopenias (n = 10) and fatigue (n = 3) the most common reasons for early discontinuation. Overall response rate was 82% (complete response, 50%; unconfirmed complete response, 5%; partial response, 26%), including 80% of patients with MCL (median age, 68; seven complete responses, one partial response). Median progression-free survival (PFS) was 25.6 months. Median PFS of patients with nonblastoid variant MCL (n = 8) was 35.9 months.
Conclusion: FFR was active in MCL, indolent B-NHL, and CLL and should be studied for older patients with MCL who are not candidates for aggressive chemotherapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Evans LS, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2003;362:139–146. - PubMed
-
- Bertoni F, Zucca E, Cavalli F. Mantle cell lymphoma. Curr Opin Hematol. 2004;11:411–418. - PubMed
-
- Gandhi MK, Marcus RE. Follicular lymphoma: Time for a re-think? Blood Rev. 2005;19:165–178. - PubMed
-
- Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–7023. - PubMed
-
- McLaughlin P, Hagemeister FB, Romaguera JE, et al. Fludarabine, mitoxantrone, and dexamethasone: An effective new regimen for indolent lymphoma. J Clin Oncol. 1996;14:1262–1268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous