Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics
- PMID: 20008701
- PMCID: PMC2891174
- DOI: 10.1001/archinternmed.2009.426
Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics
Abstract
Background: Randomized efficacy clinical trials conducted in research settings may not accurately reflect the benefits of tobacco dependence treatments when used in real-world clinical settings. Effectiveness trials (eg, in primary care settings) are needed to estimate the benefits of cessation treatments in real-world use.
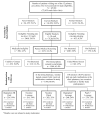
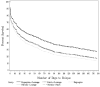
Methods: A total of 1346 primary care patients attending routine appointments were recruited by medical assistants in 12 primary care clinics. Patients were randomly assigned to 5 active pharmacotherapies: 3 monotherapies (nicotine patch, nicotine lozenge, and bupropion hydrochloride sustained release [SR]) and 2 combination therapies (patch + lozenge and bupropion SR + lozenge). Patients were referred to a telephone quit line for cessation counseling. Primary outcomes included 7-day point prevalence abstinence at 1 week, 8 weeks, and 6 months after quitting and number of days to relapse.
Results: Among 7128 eligible smokers (> or =10 cigarettes per day) attending routine primary care appointments, 1346 (18.9%) were enrolled in the study. Six-month abstinence rates for the 5 active pharmacotherapies were the following: bupropion SR, 16.8%; lozenge, 19.9%; patch, 17.7%; patch + lozenge, 26.9%; and bupropion SR + lozenge, 29.9%. Bupropion SR + lozenge was superior to all of the monotherapies (odds ratio, 0.46-0.56); patch + lozenge was superior to patch and bupropion monotherapies (odds ratio, 0.56 and 0.54, respectively).
Conclusions: One in 5 smokers attending a routine primary care appointment was willing to make a serious quit attempt that included evidence-based counseling and medication. In this comparative effectiveness study of 5 tobacco dependence treatments, combination pharmacotherapy significantly increased abstinence compared with monotherapies. Provision of free cessation medications plus quit line counseling arranged in the primary care setting holds promise for assisting large numbers of smokers to quit. Trial Registration clinicaltrials.gov Identifier: NCT00296647.
Figures
References
-
- Centers for Disease Control and Prevention. Annual smoking attributable mortality, years of potential life lost, and productivity losses — United States, 1997-2001. MMWR Morb Mortal Wkly Rep. 2005;54(25):625–628. - PubMed
-
- Giovino GA. The tobacco epidemic in the United States. Am J Prev Med. 2007;33(6 Suppl):S318–26. - PubMed
-
- Thorne SL, Malarcher A, Maurice E, Caraballa R. Cigarette smoking among adults - - United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(45):1221–1226. - PubMed
-
- Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: USDHHS, U.S. Public Health Service; 2008.
-
- Conroy MB, Majchrzak NE, Regan S, Silverman CB, Schneider LI, Rigotti NA. The association between patient-reported receipt of tobacco intervention at a primary care visit and smokers' satisfaction with their health care. Nicotine Tob Res. 2005;7(Suppl 1):S29–S34. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials