Effectiveness and cost-effectiveness of vaccination against pandemic influenza (H1N1) 2009
- PMID: 20008759
- PMCID: PMC3250217
- DOI: 10.7326/0003-4819-151-12-200912150-00157
Effectiveness and cost-effectiveness of vaccination against pandemic influenza (H1N1) 2009
Abstract
Background: Decisions on the timing and extent of vaccination against pandemic (H1N1) 2009 virus are complex.
Objective: To estimate the effectiveness and cost-effectiveness of pandemic influenza (H1N1) vaccination under different scenarios in October or November 2009.
Design: Compartmental epidemic model in conjunction with a Markov model of disease progression.
Data sources: Literature and expert opinion.
Target population: Residents of a major U.S. metropolitan city with a population of 8.3 million.
Time horizon: Lifetime.
Perspective: Societal.
Interventions: Vaccination in mid-October or mid-November 2009.
Outcome measures: Infections and deaths averted, costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness.
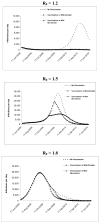
Results of base-case analysis: Assuming each primary infection causes 1.5 secondary infections, vaccinating 40% of the population in October or November would be cost-saving. Vaccination in October would avert 2051 deaths, gain 69 679 QALYs, and save $469 million compared with no vaccination; vaccination in November would avert 1468 deaths, gain 49 422 QALYs, and save $302 million.
Results of sensitivity analysis: Vaccination is even more cost-saving if longer incubation periods, lower rates of infectiousness, or increased implementation of nonpharmaceutical interventions delay time to the peak of the pandemic. Vaccination saves fewer lives and is less cost-effective if the epidemic peaks earlier than mid-October.
Limitations: The model assumed homogenous mixing of case-patients and contacts; heterogeneous mixing would result in faster initial spread, followed by slower spread. Additional costs and savings not included in the model would make vaccination more cost-saving.
Conclusion: Earlier vaccination against pandemic (H1N1) 2009 prevents more deaths and is more cost-saving. Complete population coverage is not necessary to reduce the viral reproductive rate sufficiently to help shorten the pandemic.
Primary funding source: Agency for Healthcare Research and Quality and National Institute on Drug Abuse.
Figures

Comment in
-
The modern crystal ball: influenza forecasting with mathematical models.Ann Intern Med. 2009 Dec 15;151(12):886-7. doi: 10.7326/0003-4819-151-12-200912150-00154. Ann Intern Med. 2009. PMID: 20008763 No abstract available.
Summary for patients in
-
Summaries for patients. Assessing the best way to prevent spread of influenza.Ann Intern Med. 2009 Dec 15;151(12):I31. doi: 10.7326/0003-4819-151-12-200912150-00161. Ann Intern Med. 2009. PMID: 20008744 No abstract available.
References
-
- World Health Organization. [Accessed: 18 August, 2009];Pandemic (H1N1) 2009 - update 62. 2009 August 13; http://www.who.int/csr/don/2009_08_19/en/index.html.
-
- World Health Organization. [Accessed: July 28, 2009];World now at the start of the 2009 influenza pandemic. 2009 June 11; http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6....
-
- Bureau of Vital Statistics - New York City Department of Health and Mental Hygiene. [Accessed: 17 July, 2009];SUMMARY OF VITAL STATISTICS, THE CITY OF NEW YORK. 2007 http://www.nyc.gov/html/doh/downloads/pdf/vs/2007sum.pdf.
-
- Centers for Disease Control and Prevention. Serum Cross-Reactive Antibody Response to a Novel Influenza A (H1N1) Virus After Vaccination with Seasonal Influenza Vaccine. Morb Mortal Wkly Rep. 2009;58(19):521–24. - PubMed
-
- Centers for Disease Control and Prevention. Briefing on Public Health Investigation of Human Cases of H1N1 Flu (Swine Flu) 2009 http://www.cdc.gov/media/transcripts/2009/t090501.htm Accessed.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
