Effectiveness and cost-effectiveness of expanded antiviral prophylaxis and adjuvanted vaccination strategies for an influenza A (H5N1) pandemic
- PMID: 20008760
- PMCID: PMC3428215
- DOI: 10.7326/0003-4819-151-12-200912150-00156
Effectiveness and cost-effectiveness of expanded antiviral prophylaxis and adjuvanted vaccination strategies for an influenza A (H5N1) pandemic
Abstract
Background: The pandemic potential of influenza A (H5N1) virus is a prominent public health concern of the 21st century.
Objective: To estimate the effectiveness and cost-effectiveness of alternative pandemic (H5N1) mitigation and response strategies.
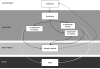
Design: Compartmental epidemic model in conjunction with a Markov model of disease progression.
Data sources: Literature and expert opinion.
Target population: Residents of a U.S. metropolitan city with a population of 8.3 million.
Time horizon: Lifetime.
Perspective: Societal.
Interventions: 3 scenarios: 1) vaccination and antiviral pharmacotherapy in quantities similar to those currently available in the U.S. stockpile (stockpiled strategy), 2) stockpiled strategy but with expanded distribution of antiviral agents (expanded prophylaxis strategy), and 3) stockpiled strategy but with adjuvanted vaccine (expanded vaccination strategy). All scenarios assumed standard nonpharmaceutical interventions.
Outcome measures: Infections and deaths averted, costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness.
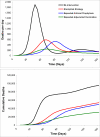
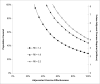
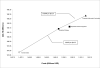
Results of base-case analysis: Expanded vaccination was the most effective and cost-effective of the 3 strategies, averting 68% of infections and deaths and gaining 404 030 QALYs at $10 844 per QALY gained relative to the stockpiled strategy.
Results of sensitivity analysis: Expanded vaccination remained incrementally cost-effective over a wide range of assumptions.
Limitations: The model assumed homogenous mixing of cases and contacts; heterogeneous mixing would result in faster initial spread, followed by slower spread. We did not model interventions for children or older adults; the model is not designed to target interventions to specific groups.
Conclusion: Expanded adjuvanted vaccination is an effective and cost-effective mitigation strategy for an influenza A (H5N1) pandemic. Expanded antiviral prophylaxis can help delay the pandemic while additional strategies are implemented.
Primary funding source: National Institutes of Health and Agency for Healthcare Research and Quality.
Figures

Comment in
-
The modern crystal ball: influenza forecasting with mathematical models.Ann Intern Med. 2009 Dec 15;151(12):886-7. doi: 10.7326/0003-4819-151-12-200912150-00154. Ann Intern Med. 2009. PMID: 20008763 No abstract available.
Summary for patients in
-
Summaries for patients. Assessing the best way to prevent spread of influenza.Ann Intern Med. 2009 Dec 15;151(12):I31. doi: 10.7326/0003-4819-151-12-200912150-00161. Ann Intern Med. 2009. PMID: 20008744 No abstract available.
References
-
- World Health Organization [June 22, 2009];Epidemic and Pandemic Alert and Response (EPR): Swine Influenza. http://www.who.int/csr/disease/swineflu/en/index.html.
-
- Binder S, Levitt AM, Sacks JJ, Hughes JM. Emerging Infectious Diseases: Public Health Issues for the 21st century. Science. 1999;284(5418):1311–13. - PubMed
-
- Webster RG, Govorkova EA. H5N1 influenza--continuing evolution and spread. N Engl J Med. 2006;355(21):2174–7. - PubMed
-
- Seo SH, Hoffmann E, Webster RG. Lethal H5N1 influenza viruses escape host antiviral cytokine responses. Nat Med. 2002;8(9):950–4. - PubMed
-
- Taubenberger JK. The virulence of the 1918 pandemic influenza virus: unraveling the enigma. Arch Virol Suppl. 2005;(19):101–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
