Inhibitor resistance in the KPC-2 beta-lactamase, a preeminent property of this class A beta-lactamase
- PMID: 20008772
- PMCID: PMC2812178
- DOI: 10.1128/AAC.00693-09
Inhibitor resistance in the KPC-2 beta-lactamase, a preeminent property of this class A beta-lactamase
Abstract
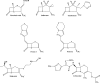
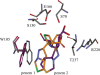
As resistance determinants, KPC beta-lactamases demonstrate a wide substrate spectrum that includes carbapenems, oxyimino-cephalosporins, and cephamycins. In addition, clinical strains harboring KPC-type beta-lactamases are often identified as resistant to standard beta-lactam-beta-lactamase inhibitor combinations in susceptibility testing. The KPC-2 carbapenemase presents a significant clinical challenge, as the mechanistic bases for KPC-2-associated phenotypes remain elusive. Here, we demonstrate resistance by KPC-2 to beta-lactamase inhibitors by determining that clavulanic acid, sulbactam, and tazobactam are hydrolyzed by KPC-2 with partition ratios (kcat/kinact ratios, where kinact is the rate constant of enzyme inactivation) of 2,500, 1,000, and 500, respectively. Methylidene penems that contain an sp2-hybridized C3 carboxylate and a bicyclic R1 side chain (dihydropyrazolo[1,5-c][1,3]thiazole [penem 1] and dihydropyrazolo[5,1-c][1,4]thiazine [penem 2]) are potent inhibitors: Km of penem 1, 0.06+/-0.01 microM, and Km of penem 2, 0.006+/-0.001 microM. We also demonstrate that penems 1 and 2 are mechanism-based inactivators, having partition ratios (kcat/kinact ratios) of 250 and 50, respectively. To understand the mechanism of inhibition by these penems, we generated molecular representations of both inhibitors in the active site of KPC-2. These models (i) suggest that penem 1 and penem 2 interact differently with active site residues, with the carbonyl of penem 2 being positioned outside the oxyanion hole and in a less favorable position for hydrolysis than that of penem 1, and (ii) support the kinetic observations that penem 2 is the better inhibitor (kinact/Km=6.5+/-0.6 microM(-1) s(-1)). We conclude that KPC-2 is unique among class A beta-lactamases in being able to readily hydrolyze clavulanic acid, sulbactam, and tazobactam. In contrast, penem-type beta-lactamase inhibitors, by exhibiting unique active site chemistry, may serve as an important scaffold for future development and offer an attractive alternative to our current beta-lactamase inhibitors.
Figures

References
-
- Bethel, C. R., A. M. Distler, M. W. Ruszczycky, M. P. Carey, P. R. Carey, A. M. Hujer, M. Taracila, M. S. Helfand, J. M. Thomson, M. Kalp, V. E. Anderson, D. A. Leonard, K. M. Hujer, T. Abe, A. M. Venkatesan, T. S. Mansour, and R. A. Bonomo. 2008. Inhibition of OXA-1 β-lactamase by penems. Antimicrob. Agents Chemother. 52:3135-3143. - PMC - PubMed
-
- Bonomo, R. A., and L. B. Rice. 1999. Inhibitor resistant class A β-lactamases. Front. Biosci. 4:e34-e41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

