What drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease?
- PMID: 20008873
- PMCID: PMC2797069
- DOI: 10.1513/pats.200907-079DP
What drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease?
Abstract
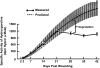
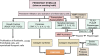
The smaller airways (<2 mm in diameter) offer little resistance in normal lungs but become the major site of obstruction in chronic obstructive pulmonary disease (COPD). We examined bronchiolar remodeling in COPD by combining quantitative histology, micro-computed tomography (CT), and gene expression studies. Volumes of bronchiolar tissue, total collagen, collagen-1, and collagen-3 were measured in lung tissue from 52 patients with different levels of COPD severity. Micro-CT was used to measure the number and lumen area of terminal bronchioles in four lungs removed before lung transplantation and in four donor lungs that served as controls. Laser capture microdissection provided 136 paired samples of bronchiolar and surrounding lung tissue from 63 patients and the gene expression of a cluster of tissue repair genes was compared. This study shows that total bronchiolar tissue decreased with progression of COPD and was associated with a reduction in total collagen and relative increase in collagen-3 over collagen-1. The micro-CT studies showed a 10-fold reduction in terminal bronchiolar number and a 100-fold reduction in lumen area. Interestingly, most genes associated with tissue accumulation during repair decreased their expression in both airways and in the surrounding lung as FEV(1) declined, but eight genes previously associated with COPD increased expression in the surrounding lung tissue. Our study shows that small airway remodeling is associated with narrowing and obliteration of the terminal bronchioles that begins before emphysematous destruction in COPD and in relation to differential expression of tissue repair genes in the airways and surrounding lung.
Figures
References
-
- Robbins SL, Cotran R. Tissue renewal and repair. In: Pathologic basis of disease, 7th ed. New York: Elsevier Saunders; 2005. pp. 87–118.
-
- Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 2009;4:435–459. - PubMed
-
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al.; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555. - PubMed
-
- Bhalla DK, Hirata F, Rishi AK, Gairola CG. Cigarette smoke, inflammation, and lung injury: a mechanistic perspective. J Toxicol Environ Health B Crit Rev 2009;12:45–64. - PubMed
-
- Di Stefano A, Turato G, Maestrelli P, Mapp CE, Ruggieri MP, Roggeri A, Boschetto P, Fabbri LM, Saetta M. Airflow limitation in chronic bronchitis is associated with T-lymphocyte and macrophage infiltration of the bronchial mucosa. Am J Respir Crit Care Med 1996;153:629–632. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
