Proceedings of the 2009 National Toxicology Program Satellite Symposium
- PMID: 20008954
- PMCID: PMC4195590
- DOI: 10.1177/0192623309354111
Proceedings of the 2009 National Toxicology Program Satellite Symposium
Abstract
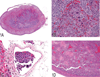
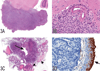
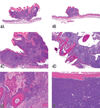
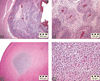
The National Toxicology Program (NTP) Satellite Symposium is a one-day meeting that is held in conjunction with the annual Society of Toxicologic Pathology (STP) meeting. The topic of the 2009 Symposium was "Tumor Pathology and INHAND (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) Nomenclature." The goal of this article is to provide summaries of each speaker's presentation, including the diagnostic or nomenclature issues that were presented, along with a few select images that were used for voting. The results of the voting process and interesting points of discussion that were raised during the presentation are also provided. A supplemental file with voting choices and voting results for each case presented at the symposium is available at http://tpx.sagepub.com/supplemental.
Figures







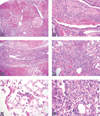

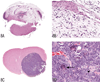
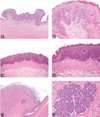
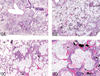



References
-
- Dagle GE, Zwicker GM, Renne RA. Morphology of spontaneous brain tumors in the rat. Vet Pathol. 1979;16:318–324. - PubMed
-
- Eastin WC, Haseman JK, Mahler JF, Burcher JR. The National Toxicology Program evaluation of genetically altered mice as predictive models for identifying carcinogens. Toxicol Pathol. 1998;26:461–473. - PubMed
-
- Frith CH, Ward JM, Turusov VS. Tumours of the liver. In Pathology of Tumours in Laboratory Animals. In: Turusov VS, Mohr U, editors. Tumours of the Mouse. 2nd ed. Vol. 2. Lyon, France: IARC Scientific Publications No. 111; 1994. pp. 223–269. - PubMed
-
- Hamlin MH, Banas DA. Adrenal gland. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, editors. In Pathology of the Fischer rat: Reference and Atlas. San Diego, CA: Academic Press, Inc; 1990. pp. 501–518.
-
- Kleihues P, Cavenee WK, editors. Pathology and genetics of tumours of the nervous system, Lyon, France: IARC Press; 2000. World Health Organization classification of tumours; pp. 1–314.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

