Imaging and quantitation of cannabinoid CB1 receptors in human and monkey brains using (18)F-labeled inverse agonist radioligands
- PMID: 20008988
- PMCID: PMC2997525
- DOI: 10.2967/jnumed.109.067074
Imaging and quantitation of cannabinoid CB1 receptors in human and monkey brains using (18)F-labeled inverse agonist radioligands
Abstract
We recently demonstrated that (11)C-MePPEP, a PET ligand for CB(1) receptors, has such high uptake in the human brain that it can be imaged for 210 min and that receptor density can be quantified as distribution volume (V(T)) using the gold standard of compartmental modeling. However, (11)C-MePPEP had relatively poor retest and intersubject variabilities, which were likely caused by errors in the measurements of radioligand in plasma at low concentrations by 120 min. We sought to find an analog of (11)C-MePPEP that would provide more accurate plasma measurements. We evaluated several promising analogs in the monkey brain and chose the (18)F-di-deutero fluoromethoxy analog ((18)F-FMPEP-d(2)) to evaluate further in the human brain.
Methods: (11)C-FMePPEP, (18)F-FEPEP, (18)F-FMPEP, and (18)F-FMPEP-d(2) were studied in 5 monkeys with 10 PET scans. We calculated V(T) using compartmental modeling with serial measurements of unchanged parent radioligand in arterial plasma and radioactivity in the brain. Nonspecific binding was determined by administering a receptor-saturating dose of rimonabant, an inverse agonist at the CB(1) receptor. Nine healthy human subjects participated in 17 PET scans using (18)F-FMPEP-d(2), with 8 subjects having 2 PET scans to assess retest variability. To identify sources of error, we compared intersubject and retest variability of brain uptake, arterial plasma measurements, and V(T).
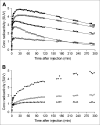
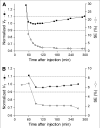
Results: (18)F-FMPEP-d(2) had high uptake in the monkey brain, with greater than 80% specific binding, and yielded less radioactivity uptake in bone than did (18)F-FMPEP. High brain uptake with (18)F-FMPEP-d(2) was also observed in humans, in whom V(T) was well identified within approximately 60 min. Retest variability of plasma measurements was good (16%); consequently, V(T) had a good retest variability (14%), intersubject variability (26%), and intraclass correlation coefficient (0.89). V(T) increased after 120 min, suggesting an accumulation of radiometabolites in the brain. Radioactivity accumulated in the skull throughout the entire scan but was thought to be an insignificant source of data contamination.
Conclusion: Studies in monkeys facilitated our development and selection of (18)F-FMPEP-d(2), compared with (18)F-FMPEP, as a radioligand demonstrating high brain uptake, high percentage of specific binding, and reduced uptake in bone. Retest analysis in human subjects showed that (18)F-FMPEP-d(2) has greater precision and accuracy than (11)C-MePPEP, allowing smaller sample sizes to detect a significant difference between groups.
Figures




References
-
- Van Laere K. In vivo imaging of the endocannabinoid system: a novel window to a central modulatory mechanism in humans. Eur J Nucl Med Mol Imaging. 2007;34:1719–1726. - PubMed
-
- Hashimoto K, Inoue O, Suzuki K, Yamasaki T, Kojima M. Deuterium isotope effect of [11C1]N,N-dimethylphenethyl-amine-a,a-d2; reduction in metabolic trapping rate in brain. Int J Rad Appl Instrum B. 1986;13:79–80. - PubMed
-
- Schou M, Halldin C, Sovago J, et al. PET evaluation of novel radiofluorinated reboxetine analogs as norepinephrine transporter probes in the monkey brain. Synapse. 2004;53:57–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
