PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM in human islets of Langerhans
- PMID: 20009023
- PMCID: PMC2838523
- DOI: 10.1152/ajpendo.00630.2009
PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM in human islets of Langerhans
Abstract
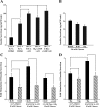
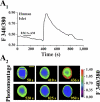
Potential insulin secretagogue properties of an acetoxymethyl ester of a cAMP analog (8-pCPT-2'-O-Me-cAMP-AM) that activates the guanine nucleotide exchange factors Epac1 and Epac2 were assessed using isolated human islets of Langerhans. RT-QPCR demonstrated that the predominant variant of Epac expressed in human islets was Epac2, although Epac1 was detectable. Under conditions of islet perifusion, 8-pCPT-2'-O-Me-cAMP-AM (10 microM) potentiated first- and second-phase 10 mM glucose-stimulated insulin secretion (GSIS) while failing to influence insulin secretion measured in the presence of 3 mM glucose. The insulin secretagogue action of 8-pCPT-2'-O-Me-cAMP-AM was associated with depolarization and an increase of [Ca(2+)](i) that reflected both Ca(2+) influx and intracellular Ca(2+) mobilization in islet beta-cells. As expected for an Epac-selective cAMP analog, 8-pCPT-2'-O-Me-cAMP-AM (10 microM) failed to stimulate phosphorylation of PKA substrates CREB and Kemptide in human islets. Furthermore, 8-pCPT-2'-O-Me-cAMP-AM (10 microM) had no significant ability to activate AKAR3, a PKA-regulated biosensor expressed in human islet cells by viral transduction. Unexpectedly, treatment of human islets with an inhibitor of PKA activity (H-89) or treatment with a cAMP antagonist that blocks PKA activation (Rp-8-CPT-cAMPS) nearly abolished the action of 8-pCPT-2'-O-Me-cAMP-AM to potentiate GSIS. It is concluded that there exists a permissive role for PKA activity in support of human islet insulin secretion that is both glucose dependent and Epac regulated. This permissive action of PKA may be operative at the insulin secretory granule recruitment, priming, and/or postpriming steps of Ca(2+)-dependent exocytosis.
Figures
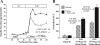

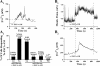
Similar articles
-
Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM.Islets. 2009 Nov-Dec;1(3):260-5. doi: 10.4161/isl.1.3.9645. Islets. 2009. PMID: 21099281 Free PMC article.
-
Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells: studies with 8-pCPT-2'-O-Me-cAMP-AM.J Biol Chem. 2009 Apr 17;284(16):10728-36. doi: 10.1074/jbc.M900166200. Epub 2009 Feb 25. J Biol Chem. 2009. PMID: 19244230 Free PMC article.
-
Phospholipase C-ε links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans.Islets. 2011 May-Jun;3(3):121-8. doi: 10.4161/isl.3.3.15507. Epub 2011 May 1. Islets. 2011. PMID: 21478675 Free PMC article.
-
Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors.Cell Signal. 2008 Jan;20(1):10-20. doi: 10.1016/j.cellsig.2007.07.009. Epub 2007 Jul 25. Cell Signal. 2008. PMID: 17716863 Free PMC article. Review.
-
Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell.Diabetes. 2004 Jan;53(1):5-13. doi: 10.2337/diabetes.53.1.5. Diabetes. 2004. PMID: 14693691 Free PMC article. Review.
Cited by
-
Glucose Signaling Is Connected to Chromosome Segregation Through Protein Kinase A Phosphorylation of the Dam1 Kinetochore Subunit in Saccharomycescerevisiae.Genetics. 2019 Feb;211(2):531-547. doi: 10.1534/genetics.118.301727. Epub 2018 Dec 13. Genetics. 2019. PMID: 30546002 Free PMC article.
-
Synthesis, characterization and pharmacodynamics of vitamin-B(12)-conjugated glucagon-like peptide-1.ChemMedChem. 2013 Apr;8(4):582-6. doi: 10.1002/cmdc.201200461. Epub 2012 Nov 30. ChemMedChem. 2013. PMID: 23203941 Free PMC article.
-
Cyclic AMP dynamics in the pancreatic β-cell.Ups J Med Sci. 2012 Nov;117(4):355-69. doi: 10.3109/03009734.2012.724732. Epub 2012 Sep 13. Ups J Med Sci. 2012. PMID: 22970724 Free PMC article. Review.
-
Pancreatic β-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A.Diabetes. 2013 Aug;62(8):2796-807. doi: 10.2337/db12-1394. Epub 2013 Apr 11. Diabetes. 2013. PMID: 23578994 Free PMC article.
-
Ghrelin attenuates cAMP-PKA signaling to evoke insulinostatic cascade in islet β-cells.Diabetes. 2011 Sep;60(9):2315-24. doi: 10.2337/db11-0368. Epub 2011 Jul 25. Diabetes. 2011. PMID: 21788571 Free PMC article.
References
-
- Allen MD, Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun 348: 716–721, 2006 - PubMed
-
- Barg S, Huang P, Eliasson L, Nelson DJ, Obermüller S, Rorsman P, Thévenod F, Renström E. Priming of insulin granules for exocytosis by granular Cl− uptake and acidification. J Cell Sci 114: 2145–2154, 2001 - PubMed
-
- Bode HP, Moormann B, Dabew R, Göke B. Glucagon-like peptide 1 elevates cytosolic calcium in pancreatic beta-cells independently of protein kinase A. Endocrinology 140: 3919–3927, 1999 - PubMed
-
- Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

