Multiple extrathymic precursors contribute to T-cell development with different kinetics
- PMID: 20009033
- PMCID: PMC2826228
- DOI: 10.1182/blood-2009-07-230821
Multiple extrathymic precursors contribute to T-cell development with different kinetics
Abstract
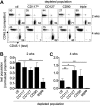
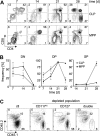
T-cell development in the thymus depends on continuous supply of T-cell progenitors from bone marrow (BM). Several extrathymic candidate progenitors have been described that range from multipotent cells to lymphoid cell committed progenitors and even largely T-lineage committed precursors. However, the nature of precursors seeding the thymus under physiologic conditions has remained largely elusive and it is not known whether there is only one physiologic T-cell precursor population or many. Here, we used a competitive in vivo assay based on depletion rather than enrichment of classes of BM-derived precursor populations, thereby only minimally altering physiologic precursor ratios to assess the contribution of various extrathymic precursors to T-lineage differentiation. We found that under these conditions multiple precursors, belonging to both multipotent progenitor (MPP) and common lymphoid progenitor (CLP) subsets have robust T-lineage potential. However, differentiation kinetics of different precursors varied considerably, which might ensure continuous thymic output despite gated importation of extrathymic precursors. In conclusion, our data suggest that the thymus functions to impose T-cell fate on any precursor capable of filling the limited number of progenitor niches.
Figures




References
-
- Allman DM, Sambandam A, Kim S, et al. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4(2):168–174. - PubMed
-
- Wada H, Masuda K, Satoh R, et al. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452(7188):768–772. - PubMed
-
- Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452(7188):764–767. - PubMed
-
- Sambandam A, Maillard I, Zediak VP, et al. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6(7):663–670. - PubMed
-
- Adolfsson J, Borge OJ, Bryder D, et al. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15(4):659–669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

