Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial
- PMID: 20009055
- PMCID: PMC2902875
- DOI: 10.1001/jama.2009.1866
Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial
Abstract
Context: Amyloid-beta peptide (Abeta(42)) has been implicated in the pathogenesis of Alzheimer disease (AD). Tarenflurbil, a selective Abeta(42)-lowering agent, demonstrated encouraging results on cognitive and functional outcomes among mildly affected patients in an earlier phase 2 trial.
Objective: To determine the efficacy, safety, and tolerability of tarenflurbil.
Design, setting, and patients: A multicenter, randomized, double-blind, placebo-controlled trial enrolling patients with mild AD was conducted at 133 trial sites in the United States between February 21, 2005, and April 30, 2008. Concomitant treatment with cholinesterase inhibitors or memantine was permitted.
Intervention: Tarenflurbil, 800 mg, or placebo, administered twice a day.
Main outcome measures: Co-primary efficacy end points were the change from baseline to month 18 in total score on the subscale of the Alzheimer Disease Assessment Scale-Cognitive Subscale (ADAS-Cog, 80-point version) and Alzheimer Disease Cooperative Studies-activities of daily living (ADCS-ADL) scale. Additional prespecified slope analyses explored the possibility of disease modification.
Results: Of the 1684 participants randomized, 1649 were included in the analysis, and 1046 completed the trial. Tarenflurbil had no beneficial effect on the co-primary outcomes (difference in change from baseline to month 18 vs placebo, based on least squares means: 0.1 for ADAS-Cog; 95% CI, -0.9 to 1.1; P = .86 and -0.5 for ADCS-ADL; 95% CI, -1.9 to 0.9; P = .48) using an intent-to-treat analysis. No significant differences occurred in the secondary outcomes. The ADAS-Cog score decreased by 7.1 points over 18 months. The tarenflurbil group had a small increase in frequency of dizziness, anemia, and infections.
Conclusion: Tarenflurbil did not slow cognitive decline or the loss of activities of daily living in patients with mild AD.
Trial registration: clinicaltrials.gov Identifier: NCT00105547.
Figures
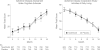
Comment in
-
Late-life dementias: does this unyielding global challenge require a broader view?JAMA. 2009 Dec 16;302(23):2593-4. doi: 10.1001/jama.2009.1863. JAMA. 2009. PMID: 20009062 Free PMC article. No abstract available.
-
Tarenflurbil: mechanisms and myths.Arch Neurol. 2010 Jun;67(6):750-2. doi: 10.1001/archneurol.2010.94. Arch Neurol. 2010. PMID: 20558395 Free PMC article. No abstract available.
-
Tarenflurbil in patients with mild Alzheimer's disease.Curr Neurol Neurosci Rep. 2010 Sep;10(5):336-7. doi: 10.1007/s11910-010-0130-6. Curr Neurol Neurosci Rep. 2010. PMID: 20571933 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

