Plasma membrane expression of gonadotropin-releasing hormone receptors: regulation by peptide and nonpeptide antagonists
- PMID: 20009083
- PMCID: PMC2830153
- DOI: 10.1210/me.2009-0343
Plasma membrane expression of gonadotropin-releasing hormone receptors: regulation by peptide and nonpeptide antagonists
Abstract
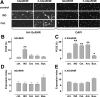
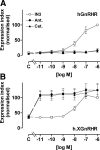
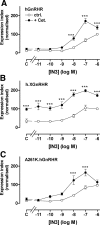
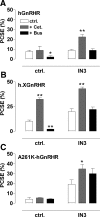
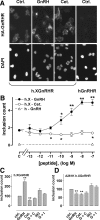
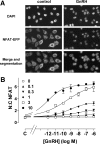
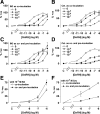
Gonadotropin-releasing hormone acts via cell surface receptors but most human (h) GnRH receptors (GnRHRs) are intracellular. A membrane-permeant nonpeptide antagonist [(2S)-2-[5-[2-(2-axabicyclo[2.2.2]oct-2-yl)-1,1-dimethy-2-oxoethyl]-2-(3,5-dimethylphenyl)-1H-indol-3-yl]-N-(2-pyridin-4-ylethyl)propan-1-amine (IN3)] increases hGnRHR expression at the surface, apparently by facilitating its exit from the endoplasmic reticulum. Here we have quantified GnRHR by automated imaging in HeLa cells transduced with adenovirus expressing hemagglutinin-tagged GnRHR. Consistent with an intracellular site of action, IN3 increases cell surface hGnRHR, and this effect is not blocked or mimicked by membrane-impermeant peptide antagonists [Ac-D2Nal-D4Cpa-D3Pal-Ser-Tyr-d-Cit-Leu-Arg-Pro-d-Ala-NH(2) (cetrorelix) and antide]. However, when the C-terminal tail of a Xenopus (X) GnRHR was added (h.XGnRHR) to increase expression, both peptides further increased cell surface GnRHR. Cetrorelix also synergized with IN3 to increase expression of hGnRHR and a G-protein coupling-deficient mutant (A261K-hGnRHR). Cetrorelix also increased cell surface expression of hGnRHR, h.XGnRHR, and mouse GnRHR in gonadotrope-lineage LbetaT2 cells, and in HeLa cells it slowed h.XGnRHR internalization (measured by receptor-mediated antihemagglutinin uptake). Thus cetrorelix has effects other than GnRHR blockade; it acts as an inverse agonist in internalization assays, supporting the potential importance of ligand-biased efficacy at GnRHR. We also developed an imaging assay for GnRH function based on Ca(2+)-dependent nuclear translocation of a nuclear factor of activated T cells reporter. Using this in HeLa and LbetaT2 cells, IN3 and cetrorelix behaved as competitive antagonists when coincubated with GnRH, and long-term pretreatment (16 h) with IN3 reduced its effectiveness as an inhibitor whereas pretreatment with cetrorelix increased its inhibitory effect. This distinction between peptide and nonpeptide antagonists may prove important for therapeutic applications of GnRH antagonists.
Figures
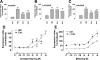

References
-
- Conn PM, Huckle WR, Andrews WV, McArdle CA 1987 The molecular mechanism of action of gonadotropin releasing hormone (GnRH) in the pituitary. Recent Prog Horm Res 43:29–68 - PubMed
-
- Stojilkovic SS, Catt KJ 1995 Expression and signal transduction pathways of gonadotropin-releasing hormone receptors. Recent Prog Horm Res 50:161–205 - PubMed
-
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR 2004 Gonadotropin-releasing hormone receptors. Endocr Rev 25:235–275 - PubMed
-
- Cheng CK, Leung PC 2005 Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr Rev 26:283–306 - PubMed
-
- Rispoli LA, Nett TM 2005 Pituitary gonadotropin-releasing hormone receptor: structure, distribution and regulation of expression. Anim Reprod Sci 88:57–74 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

