Hyperglycemia impairs proteasome function by methylglyoxal
- PMID: 20009088
- PMCID: PMC2828656
- DOI: 10.2337/db08-1565
Hyperglycemia impairs proteasome function by methylglyoxal
Abstract
Objective: The ubiquitin-proteasome system is the main degradation machinery for intracellularly altered proteins. Hyperglycemia has been shown to increase intracellular levels of the reactive dicarbonyl methylglyoxal (MGO) in cells damaged by diabetes, resulting in modification of proteins and alterations of their function. In this study, the influence of MGO-derived advanced glycation end product (AGE) formation on the activity of the proteasome was investigated in vitro and in vivo.
Research design and methods: MGO-derived AGE modification of proteasome subunits was analyzed by mass spectrometry, immunoprecipitation, and Western blots. Proteasome activity was analyzed using proteasome-specific fluorogenic substrates. Experimental models included bovine retinal endothelial cells, diabetic Ins2(Akita) mice, glyoxalase 1 (GLO1) knockdown mice, and streptozotocin (STZ)-injected diabetic mice.
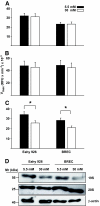
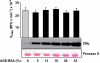
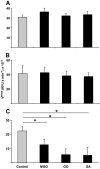
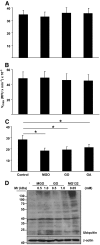
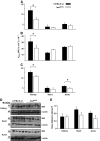
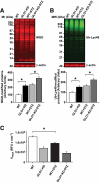
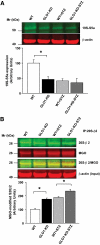
Results: In vitro incubation with MGO caused adduct formation on several 20S proteasomal subunit proteins. In cultured endothelial cells, the expression level of the catalytic 20S proteasome subunit was not altered but proteasomal chymotrypsin-like activity was significantly reduced. In contrast, levels of regulatory 19S proteasomal proteins were decreased. In diabetic Ins2(Akita), STZ diabetic, and nondiabetic and diabetic G101 knockdown mice, chymotrypsin-like activity was also reduced and MGO modification of the 20S-beta2 subunit was increased.
Conclusions: Hyperglycemia-induced formation of MGO covalently modifies the 20S proteasome, decreasing its activity in the diabetic kidney and reducing the polyubiquitin receptor 19S-S5a. The results indicate a new link between hyperglycemia and impairment of cell functions.
Figures
References
-
- Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813– 820 - PubMed
-
- Thornalley PJ: Dicarbonyl intermediates in the maillard reaction. Ann N Y Acad Sci 2005; 1043: 111– 117 - PubMed
-
- Karachalias N, Babaei-Jadidi R, Ahmed N, Thornalley PJ: Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rats. Biochem Soc Trans 2003; 31: 1423– 1425 - PubMed
-
- Miyata T, van Ypersele de Strihou C, Imasawa T, Yoshino A, Ueda Y, Ogura H, Kominami K, Onogi H, Inagi R, Nangaku M, Kurokawa K: Glyoxalase I deficiency is associated with an unusual level of advanced glycation end products in a hemodialysis patient. Kidney Int 2001; 60: 2351– 2359 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

