Rapid identification of myocardial infarction risk associated with diabetes medications using electronic medical records
- PMID: 20009093
- PMCID: PMC2827502
- DOI: 10.2337/dc09-1506
Rapid identification of myocardial infarction risk associated with diabetes medications using electronic medical records
Abstract
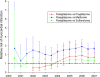
OBJECTIVE To assess the ability to identify potential association(s) of diabetes medications with myocardial infarction using usual care clinical data obtained from the electronic medical record. RESEARCH DESIGN AND METHODS We defined a retrospective cohort of patients (n = 34,253) treated with a sulfonylurea, metformin, rosiglitazone, or pioglitazone in a single academic health care network. All patients were aged >18 years with at least one prescription for one of the medications between 1 January 2000 and 31 December 2006. The study outcome was acute myocardial infarction requiring hospitalization. We used a cumulative temporal approach to ascertain the calendar date for earliest identifiable risk associated with rosiglitazone compared with that for other therapies. RESULTS Sulfonylurea, metformin, rosiglitazone, or pioglitazone therapy was prescribed for 11,200, 12,490, 1,879, and 806 patients, respectively. A total of 1,343 myocardial infarctions were identified. After adjustment for potential myocardial infarction risk factors, the relative risk for myocardial infarction with rosiglitazone was 1.3 (95% CI 1.1-1.6) compared with sulfonylurea, 2.2 (1.6-3.1) compared with metformin, and 2.2 (1.5-3.4) compared with pioglitazone. Prospective surveillance using these data would have identified increased risk for myocardial infarction with rosiglitazone compared with metformin within 18 months of its introduction with a risk ratio of 2.1 (95% CI 1.2-3.8). CONCLUSIONS Our results are consistent with a relative adverse cardiovascular risk profile for rosiglitazone. Our use of usual care electronic data sources from a large hospital network represents an innovative approach to rapid safety signal detection that may enable more effective postmarketing drug surveillance.
Figures
References
-
- Misbin RI: Lessons from the Avandia controversy: a new paradigm for the development of drugs to treat type 2 diabetes. Diabetes Care 2007;30:3141–3144 - PubMed
-
- Davis RL, Kolczak M, Lewis E, Nordin J, Goodman M, Shay DK, Platt R, Black S, Shinefield H, Chen RT: Active surveillance of vaccine safety: a system to detect early signs of adverse events. Epidemiology 2005;16:336–341 - PubMed
-
- Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471 - PubMed
-
- Singh S, Loke YK, Furberg CD: Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 2007;298:1189–1195 - PubMed
-
- Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ: RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373:2125–2135 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

