Targeting pericytes with a PDGF-B aptamer in human ovarian carcinoma models
- PMID: 20009575
- PMCID: PMC3155813
- DOI: 10.4161/cbt.9.3.10635
Targeting pericytes with a PDGF-B aptamer in human ovarian carcinoma models
Abstract
Purpose: On the basis of the known role of platelet-derived growth factor (PDGF)-BB/PDGF receptor (PDGFR) beta in pericyte regulation, highly specific inhibitors of this target are needed. We tested the efficacy of a highly selective aptamer against PDGF-B with or without anti-VEGF therapy in ovarian cancer models.
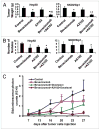
Results: Bevacizumab inhibited tumor growth by 45% and 48% in the HeyA8 and SKOV3ip1 models, respectively. AX102 had minimal effect on the HeyA8 model, but increased tumor growth in the SKOV3ip1 model. However, bevacizumab plus AX102 was more effective than bevacizumab alone, and resulted in 76-88% inhibition of tumor growth in both models. A longitudinal study in the HeyA8 model using bioluminescence imaging showed that combination of bevacizumab, AX102 and paclitaxel caused tumor reduction by 65% (based on bioluminescence imaging). In the HeyA8 model, MVD and PCNA counts were significantly reduced in the bevacizumab treatment groups, and pericyte coverage was significantly decreased in the AX102 treatment groups. In the SKOV3ip1 model, MVD and PCNA was significantly reduced in the bevacizumab treatment group, and even lower in the bevacizumab and AX102 combination treatment group.
Experimental design: The therapeutic efficacy of targeting endothelial cells (bevacizumab) and/or pericytes (PDGF-aptamer, AX102) was examined using HeyA8 and SKOV3ip1 orthotopic models of ovarian cancer metastasis. Following therapy, tumors were examined for microvessel density (MVD), proliferating cell nuclear antigen (PCNA) and vascular maturation (pericyte coverage).
Conclusions: Dual targeting of endothelial cells and pericytes holds potential as an anti-vascular therapeutic approach in ovarian carcinoma.
Figures


Comment in
-
Two is better than one: benefits of VEGF and PDGF inhibition in ovarian cancer.Cancer Biol Ther. 2010 Feb;9(3):183-5. doi: 10.4161/cbt.9.3.11117. Epub 2010 Feb 4. Cancer Biol Ther. 2010. PMID: 20083903 No abstract available.
References
-
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. - PubMed
-
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. - PubMed
-
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
