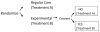Randomized clinical trials in stroke research
- PMID: 20009954
- PMCID: PMC2837939
- DOI: 10.2310/JIM.0b013e3181c9b2d4
Randomized clinical trials in stroke research
Abstract
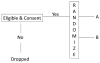
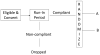
A randomized clinical trial is widely regarded as the most rigorous study design to determine the efficacy of intervention because spurious causality and bias associated with other experimental designs can be avoided. The purpose of this article is to provide clinicians and clinical researchers the types of randomized clinical trials used in stroke studies and to discuss the advantages and the limitations for each type of randomized stroke clinical trials.
Figures
References
-
- Lachin JM, Matts JP, Wei LJ. Randomization in clinical trials: Conclusions and recommendations. Controlled Clinical Trials. 1988;9:365–374. - PubMed
-
- Altman DG. Practical statistics for Medical Research. Chapman and Hall; 1991.
-
- Schulz KF, Grimes DA. Sample size calculations in randomized trials: mandatory and mystical. Lancet. 2005;365:1348–1353. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases