Na/H exchange inhibition protects newborn heart from ischemia/reperfusion injury by limiting Na+-dependent Ca2+ overload
- PMID: 20010437
- PMCID: PMC2854839
- DOI: 10.1097/FJC.0b013e3181cb599f
Na/H exchange inhibition protects newborn heart from ischemia/reperfusion injury by limiting Na+-dependent Ca2+ overload
Abstract
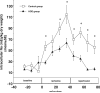
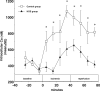
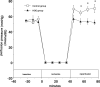
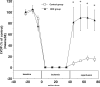
The results of the Guardian/Expedition trials demonstrate the need for more precisely controlled studies to inhibit Na/H exchange (NHE1) during ischemia/reperfusion. This is because overwhelming evidence is consistent with the hypothesis that myocardial ischemic injury results in part from increases in intracellular Na (Nai) mediated by NHE1 that in turn promote Na/Ca exchanger-mediated increases in intracellular Ca ([Ca]i) and Ca-dependent cell damage. We used a more potent and specific NHE1 inhibitor HOE 694 (HOE) to test whether inhibition of NHE1 during ischemia limits increases in Nai and [Ca]i in newborns. NMR was used to measure pHi, Nai, [Ca]i, and ATP in isolated newborn rabbit hearts. Perfusion pressure, left ventricular developed pressure, and creatine kinase were measured. HOE was added before global ischemia. Results are reported as mean +/- SE. Nai (mEq/kg dry weight) rose from 11.6 +/- 0.9 before ischemia to 114.0 +/- 16.1 at the end of ischemia and recovered to 55.2 +/- 11.8 in the control group. During ischemia and reperfusion, the corresponding values for Nai in the HOE group (63.1 +/- 8.4 and 15.9 +/- 2.5, respectively, P < 0.05) were lower than control. In the control group [Ca]i (nM/L) rose from 331 +/- 41 to 1069 +/- 71 and recovered to 814 +/- 51, whereas in the HOE group [Ca]i rose less (P < 0.05): 359 +/- 50, 607 +/- 85, and 413 +/- 40, respectively. Total creatine kinase release was significantly reduced in the HOE group. Perfusion pressure and left ventricular developed pressure also recovered significantly better in the HOE group than in the control. In conclusion, NHE1 inhibition diminishes ischemia-induced increases in Nai and therefore [Ca], and thus diminishes myocardial injury in neonatal hearts.
Figures



Similar articles
-
Ethylisopropylamiloride diminishes changes in intracellular Na, Ca and pH in ischemic newborn myocardium.J Mol Cell Cardiol. 1997 Aug;29(8):2077-86. doi: 10.1006/jmcc.1997.0442. J Mol Cell Cardiol. 1997. PMID: 9281440
-
Ischemic preconditioning: effects on pH, Na and Ca in newborn rabbit hearts during Ischemia/Reperfusion.J Mol Cell Cardiol. 1998 Mar;30(3):685-97. doi: 10.1006/jmcc.1997.0636. J Mol Cell Cardiol. 1998. PMID: 9515043
-
New Na(+)-H+ exchange inhibitor HOE 694 improves postischemic function and high-energy phosphate resynthesis and reduces Ca2+ overload in isolated perfused rabbit heart.Circulation. 1994 Jun;89(6):2787-98. doi: 10.1161/01.cir.89.6.2787. Circulation. 1994. PMID: 8205693
-
Sodium ion/hydrogen ion exchange inhibition: a new pharmacologic approach to myocardial ischemia and reperfusion injury.J Clin Pharmacol. 1998 Oct;38(10):887-97. doi: 10.1002/j.1552-4604.1998.tb04383.x. J Clin Pharmacol. 1998. PMID: 9807968 Review.
-
Mechanisms of protection of the ischemic and reperfused myocardium by sodium-hydrogen exchange inhibition.J Thromb Thrombolysis. 1999 Jul;8(1):33-8. doi: 10.1023/a:1008990530176. J Thromb Thrombolysis. 1999. PMID: 10481212 Review.
Cited by
-
Cardioprotection and myocardial reperfusion: pitfalls to clinical application.Circ Res. 2013 Aug 2;113(4):464-77. doi: 10.1161/CIRCRESAHA.113.300765. Circ Res. 2013. PMID: 23908333 Free PMC article. Review.
-
Resveratrol attenuates the Na(+)-dependent intracellular Ca(2+) overload by inhibiting H(2)O(2)-induced increase in late sodium current in ventricular myocytes.PLoS One. 2012;7(12):e51358. doi: 10.1371/journal.pone.0051358. Epub 2012 Dec 13. PLoS One. 2012. PMID: 23272101 Free PMC article.
-
Enterohemorrhagic Escherichia coli infection stimulates Shiga toxin 1 macropinocytosis and transcytosis across intestinal epithelial cells.Am J Physiol Cell Physiol. 2011 Nov;301(5):C1140-9. doi: 10.1152/ajpcell.00036.2011. Epub 2011 Aug 10. Am J Physiol Cell Physiol. 2011. PMID: 21832249 Free PMC article.
-
Ischemic factor-induced increases in cerebral microvascular endothelial cell Na/H exchange activity and abundance: evidence for involvement of ERK1/2 MAP kinase.Am J Physiol Cell Physiol. 2014 May 15;306(10):C931-42. doi: 10.1152/ajpcell.00021.2013. Epub 2014 Mar 19. Am J Physiol Cell Physiol. 2014. PMID: 24647544 Free PMC article.
-
Hydroxytyrosol protects against myocardial ischemia reperfusion injury by inhibiting mitochondrial permeability transition pore opening.Exp Ther Med. 2019 Jan;17(1):671-678. doi: 10.3892/etm.2018.7016. Epub 2018 Nov 27. Exp Ther Med. 2019. PMID: 30651849 Free PMC article.
References
-
- Imura H, Caputo M, Parry A, Pawade A, Angelini G, Suleiman M. Age-dependent and hypoxia-related differences in myocardial protection during pediatric open heart surgery. Circulation. 2001;103:1551–6. - PubMed
-
- Karimi M, Wang LX, Hammel JM, Mascio CE, Abdulhamid M, Barner EW, Scholz TD, Segar JL, Li WG, Niles SD, Caldarone CA. Neonatal vulnerability to ischemia and reperfusion: Cardioplegic arrest causes greater myocardial apoptosis in neonatal lambs than in mature lambs. J Thorac Cardiovasc Surg. 2004 Feb;127(2):490–7. - PubMed
-
- Myers M, Mathur S, Li G, Karmazyn M. Sodium-hydrogen exchange inhibitors improve postischaemic recovery of function in the perfused rabbit heart. Cardiovasc Res. 1995;29:209–214. - PubMed
-
- Murphy E, Perlman M, London RE, Steenbergen C. Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circ. Res. 1991;68:1250–1258. - PubMed
-
- Scholz W, Albus U. Potential of selective sodium-hydrogen exchange inhibitors in cardiovascular therapy. Cardiovasc Res. 1995;29:184–188. - PubMed

