Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement
- PMID: 20010550
- PMCID: PMC3055366
- DOI: 10.1038/npp.2009.193
Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement
Abstract
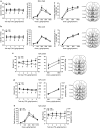
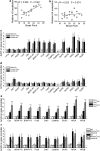
The regulation of gene expression in the brain reward regions is known to contribute to the pathogenesis and persistence of drug addiction. Increasing evidence suggests that the regulation of gene transcription is mediated by epigenetic mechanisms that alter the chromatin structure at specific gene promoters. To better understand the involvement of epigenetic regulation in drug reinforcement properties, rats were subjected to cocaine self-administration paradigm. Daily histone deacetylase (HDAC) inhibitor infusions in the shell of the nucleus accumbens (NAc) caused an upward shift in the dose-response curve under fixed-ratio schedule and increased the break point under progressive-ratio schedule, indicating enhanced motivation for self-administered drug. The effect of the HDAC inhibitor is attributed to the increased elevation of histone acetylation induced by chronic, but not acute, cocaine experience. In contrast, neutralizing the chronic cocaine-induced increase in histone modification by the bilateral overexpression of HDAC4 in the NAc shell reduced drug motivation. The association between the motivation for cocaine and the transcriptional activation of addiction-related genes by H3 acetylation in the NAc shell was analyzed. Among the genes activated by chronic cocaine experiences, the expression of CaMKIIalpha, but not CaMKIIbeta, correlated positively with motivation for the drug. Lentivirus-mediated shRNA knockdown experiments showed that CaMKIIalpha, but not CaMKIIbeta, in the NAc shell is essential for the maintenance of motivation to self-administered cocaine. These findings suggest that chronic drug-use-induced transcriptional activation of genes, such as CaMKIIalpha, modulated by H3 acetylation in the NAc is a critical regulatory mechanism underlying motivation for drug reinforcement.
Figures





References
-
- Ahmed S, Koob G. Vertical shifts in dose–injection curves reflect reward allostasis, not sensitization. Psychopharmacology. 2004;171:354–355.
-
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. - PubMed
-
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. - PubMed
-
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. - PubMed
-
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

