Antagonism of Beclin 1-dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1
- PMID: 20010695
- PMCID: PMC2830692
- DOI: 10.1038/emboj.2009.369
Antagonism of Beclin 1-dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1
Abstract
In addition to mitochondria, BCL-2 is located at the endoplasmic reticulum (ER) where it is a constituent of several distinct complexes. Here, we identify the BCL-2-interacting protein at the ER, nutrient-deprivation autophagy factor-1 (NAF-1)-a bitopic integral membrane protein whose defective expression underlies the aetiology of the neurodegenerative disorder Wolfram syndrome 2 (WFS2). NAF-1 contains a two iron-two sulphur coordinating domain within its cytosolic region, which is necessary, but not sufficient for interaction with BCL-2. NAF-1 is displaced from BCL-2 by the ER-restricted BH3-only protein BIK and contributes to regulation of BIK-initiated autophagy, but not BIK-dependent activation of caspases. Similar to BCL-2, NAF-1 is found in association with the inositol 1,4,5-triphosphate receptor and is required for BCL-2-mediated depression of ER Ca(2+) stores. During nutrient deprivation as a physiological stimulus of autophagy, BCL-2 is known to function through inhibition of the autophagy effector and tumour suppressor Beclin 1. NAF-1 is required in this pathway for BCL-2 at the ER to functionally antagonize Beclin 1-dependent autophagy. Thus, NAF-1 is a BCL-2-associated co-factor that targets BCL-2 for antagonism of the autophagy pathway at the ER.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
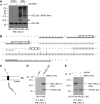
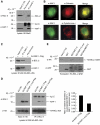

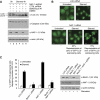

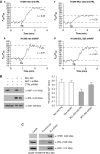
Comment in
-
Crosstalk between apoptosis and autophagy within the Beclin 1 interactome.EMBO J. 2010 Feb 3;29(3):515-6. doi: 10.1038/emboj.2009.377. EMBO J. 2010. PMID: 20125189 Free PMC article.
References
-
- Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, Ebb RG, Subramanian T, Chittenden T, Lutz RJ, Chinnadurai G (1995) Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene 11: 1921–1928 - PubMed
-
- Brady NR, Hamacher-Brady A, Yuan H, Gottlieb RA (2007) The autophagic response to nutrient deprivation in the hl-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. FEBS J 274: 3184–3197 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

