Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells
- PMID: 20010812
- PMCID: PMC2906259
- DOI: 10.1038/ncb2009
Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells
Erratum in
- Nat Cell Biol. 2011 Jun;13(6):734
Abstract
The continuing rise in atmospheric CO2 causes stomatal pores in leaves to close and thus globally affects CO2 influx into plants, water use efficiency and leaf heat stress. However, the CO2-binding proteins that control this response remain unknown. Moreover, which cell type responds to CO2, mesophyll or guard cells, and whether photosynthesis mediates this response are matters of debate. We demonstrate that Arabidopsis thaliana double-mutant plants in the beta-carbonic anhydrases betaCA1 and betaCA4 show impaired CO2-regulation of stomatal movements and increased stomatal density, but retain functional abscisic-acid and blue-light responses. betaCA-mediated CO2-triggered stomatal movements are not, in first-order, linked to whole leaf photosynthesis and can function in guard cells. Furthermore, guard cell betaca-overexpressing plants exhibit instantaneous enhanced water use efficiency. Guard cell expression of mammalian alphaCAII complements the reduced sensitivity of ca1 ca4 plants, showing that carbonic anhydrase-mediated catalysis is an important mechanism for betaCA-mediated CO2-induced stomatal closure and patch clamp analyses indicate that CO2/HCO3- transfers the signal to anion channel regulation. These findings, together with ht1-2 (ref. 9) epistasis analysis demonstrate that carbonic anhydrases function early in the CO2 signalling pathway, which controls gas-exchange between plants and the atmosphere.
Figures
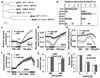


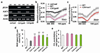

Similar articles
-
SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling.Nature. 2008 Mar 27;452(7186):487-91. doi: 10.1038/nature06608. Epub 2008 Feb 27. Nature. 2008. PMID: 18305484 Free PMC article.
-
Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2 - and ABA-induced stomatal closing.Plant J. 2015 Aug;83(4):567-81. doi: 10.1111/tpj.12916. Epub 2015 Jul 22. Plant J. 2015. PMID: 26096271 Free PMC article.
-
Distinct Cellular Locations of Carbonic Anhydrases Mediate Carbon Dioxide Control of Stomatal Movements.Plant Physiol. 2015 Oct;169(2):1168-78. doi: 10.1104/pp.15.00646. Epub 2015 Aug 4. Plant Physiol. 2015. PMID: 26243620 Free PMC article.
-
Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses.Ann Bot. 2012 Jan;109(1):5-17. doi: 10.1093/aob/mcr252. Epub 2011 Oct 12. Ann Bot. 2012. PMID: 21994053 Free PMC article. Review.
-
CO2 Sensing and CO2 Regulation of Stomatal Conductance: Advances and Open Questions.Trends Plant Sci. 2016 Jan;21(1):16-30. doi: 10.1016/j.tplants.2015.08.014. Epub 2015 Oct 5. Trends Plant Sci. 2016. PMID: 26482956 Free PMC article. Review.
Cited by
-
Dominant and recessive mutations in the Raf-like kinase HT1 gene completely disrupt stomatal responses to CO2 in Arabidopsis.J Exp Bot. 2016 May;67(11):3251-61. doi: 10.1093/jxb/erw134. Epub 2016 Mar 31. J Exp Bot. 2016. PMID: 27034327 Free PMC article.
-
Null mutants of a tomato Rho of plants exhibit enhanced water use efficiency without a penalty to yield.Proc Natl Acad Sci U S A. 2024 Jan 23;121(4):e2309006120. doi: 10.1073/pnas.2309006120. Epub 2024 Jan 8. Proc Natl Acad Sci U S A. 2024. PMID: 38190516 Free PMC article.
-
Stomatal and pavement cell density linked to leaf internal CO2 concentration.Ann Bot. 2014 Aug;114(2):191-202. doi: 10.1093/aob/mcu095. Epub 2014 May 13. Ann Bot. 2014. PMID: 24825295 Free PMC article.
-
Molecular Characterization of Carbonic Anhydrase Genes in Lotus japonicus and Their Potential Roles in Symbiotic Nitrogen Fixation.Int J Mol Sci. 2021 Jul 21;22(15):7766. doi: 10.3390/ijms22157766. Int J Mol Sci. 2021. PMID: 34360533 Free PMC article.
-
Illuminating stomatal responses to red light: establishing the role of Ci-dependent versus -independent mechanisms in control of stomatal behaviour.J Exp Bot. 2024 Nov 15;75(21):6810-6822. doi: 10.1093/jxb/erae093. J Exp Bot. 2024. PMID: 38442206 Free PMC article.
References
-
- Sellers PJ, et al. Modeling the exchanges of energy, water, and carbon between continents and the atmosphere. Science. 1997;275:502–509. - PubMed
-
- Medlyn BE, et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol. 2001;149:247–264. - PubMed
-
- LaDeau SL, Clark JS. Rising CO2 levels and the fecundity of forest trees. Science. 2001;292:95–98. - PubMed
-
- Battisti DS, Naylor RL. Historical warnings of future food insecurity with unprecedented seasonal heat. Science. 2009;323:240–244. - PubMed
-
- von Caemmerer S, et al. Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J. Exp. Bot. 2004;55:1157–1166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

