PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane
- PMID: 20010819
- PMCID: PMC2810846
- DOI: 10.1038/nn.2462
PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane
Abstract
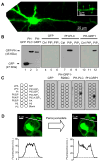
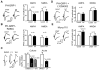
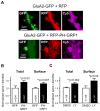
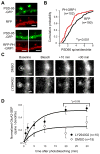
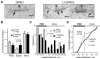
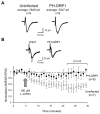
Despite their low abundance, phosphoinositides are critical regulators of intracellular signaling and membrane compartmentalization. However, little is known of phosphoinositide function at the postsynaptic membrane. Here we show that continuous synthesis and availability of phosphatidylinositol-(3,4,5)-trisphosphate (PIP(3)) at the postsynaptic terminal is necessary for sustaining synaptic function in rat hippocampal neurons. This requirement was specific for synaptic, but not extrasynaptic, AMPA receptors, nor for NMDA receptors. PIP(3) downregulation impaired PSD-95 accumulation in spines. Concomitantly, AMPA receptors became more mobile and migrated from the postsynaptic density toward the perisynaptic membrane within the spine, leading to synaptic depression. Notably, these effects were only revealed after prolonged inhibition of PIP(3) synthesis or by direct quenching of this phosphoinositide at the postsynaptic cell. Therefore, we conclude that a slow, but constant, turnover of PIP(3) at synapses is required for maintaining AMPA receptor clustering and synaptic strength under basal conditions.
Figures