The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls
- PMID: 20010917
- PMCID: PMC2839427
- DOI: 10.1038/mt.2009.281
The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls
Abstract
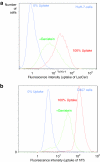
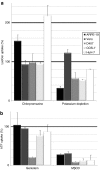
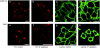
Nonviral gene complexes can enter mammalian cells through different endocytic pathways. For efficient optimization of the gene carrier it is important to profile its cellular uptake, because this largely determines its intracellular processing and subsequent transfection efficiency. Most of the current information on uptake of these gene-delivery vehicles is based on data following the use of chemical inhibitors of endocytic pathways. Here, we have performed a detailed characterization of four commonly used endocytosis inhibitors [chlorpromazine, genistein, methyl-beta-cyclodextrin (MbetaCD), and potassium depletion] on cell viability and endocytosis in five well-described cell lines. We found that chlorpromazine and to a lesser extent MbetaCD significantly decreased cell viability of some cell lines even after short incubation periods and at concentrations that are routinely used to inhibit endocytosis. Through analyzing the uptake and subcellular distribution of two fluorescent endocytic probes transferrin and lactosylceramide (LacCer) that are reported to enter cells via clathrin-dependent (CDE) and clathrin-independent (CIE) mechanisms, respectively, we showed poor specificity of these agents for inhibiting distinct endocytic pathways. Finally, we demonstrate that any inhibitory effects are highly cell line dependent. Overall, the data question the significance of performing endocytosis studies with these agents in the absence of very stringent controls.
Figures





References
-
- Wattiaux R, Laurent N, Wattiaux-De Coninck S., and , Jadot M. Endosomes, lysosomes: their implication in gene transfer. Adv Drug Deliv Rev. 2000;41:201–208. - PubMed
-
- Conner SD., and , Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. - PubMed
-
- Grimmer S, van Deurs B., and , Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002;115 Pt 14:2953–2962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

