Comparative transduction efficiency of AAV vector serotypes 1-6 in the substantia nigra and striatum of the primate brain
- PMID: 20010918
- PMCID: PMC2839442
- DOI: 10.1038/mt.2009.286
Comparative transduction efficiency of AAV vector serotypes 1-6 in the substantia nigra and striatum of the primate brain
Abstract
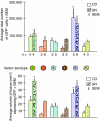
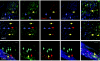
Vectors derived from adeno-associated virus (AAV) are promising candidates for neural cell transduction in vivo because they are nonpathogenic and achieve long-term transduction in the central nervous system. AAV serotype 2 (AAV2) is the most widely used AAV vector in clinical trials based largely on its ability to transduce neural cells in the rodent and primate brain. Prior work in rodents suggests that other serotypes might be more efficient; however, a systematic evaluation of vector transduction efficiency has not yet been performed in the primate brain. In this study, AAV viral vectors of serotypes 1-6 with an enhanced green-fluorescent protein (GFP) reporter gene were generated at comparable titers, and injected in equal amounts into the brains of Chlorocebus sabaeus. Vector injections were placed in the substantia nigra (SN) and the caudate nucleus (CD). One month after injection, immunohistochemistry for GFP was performed and the total number of GFP+ cells was calculated using unbiased stereology. AAV5 was the most efficient vector, not only transducing significantly more cells than any other serotype, but also transducing both NeuN+ and glial-fibrillary-acidic protein positive (GFAP+) cells. These results suggest that AAV5 is a more effective vector than AAV2 at delivering potentially therapeutic transgenes to the nigrostriatal system of the primate brain.
Figures


Similar articles
-
Cellular selectivity of AAV serotypes for gene delivery in neurons and astrocytes by neonatal intracerebroventricular injection.PLoS One. 2017 Dec 15;12(12):e0188830. doi: 10.1371/journal.pone.0188830. eCollection 2017. PLoS One. 2017. PMID: 29244806 Free PMC article.
-
Differential transduction following basal ganglia administration of distinct pseudotyped AAV capsid serotypes in nonhuman primates.Mol Ther. 2010 Mar;18(3):579-87. doi: 10.1038/mt.2009.216. Epub 2009 Sep 22. Mol Ther. 2010. PMID: 19773746 Free PMC article.
-
Gene transfer to the nigrostriatal system by hybrid herpes simplex virus/adeno-associated virus amplicon vectors.Hum Gene Ther. 1999 Oct 10;10(15):2481-94. doi: 10.1089/10430349950016825. Hum Gene Ther. 1999. PMID: 10543613
-
Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model.Brain Res. 2000 Dec 15;886(1-2):82-98. doi: 10.1016/s0006-8993(00)02915-2. Brain Res. 2000. PMID: 11119690 Review.
-
Genetic Constructs for the Control of Astrocytes' Activity.Cells. 2021 Jun 25;10(7):1600. doi: 10.3390/cells10071600. Cells. 2021. PMID: 34202359 Free PMC article. Review.
Cited by
-
A brain microvasculature endothelial cell-specific viral vector with the potential to treat neurovascular and neurological diseases.EMBO Mol Med. 2016 Jun 1;8(6):609-25. doi: 10.15252/emmm.201506078. Print 2016 Jun. EMBO Mol Med. 2016. PMID: 27137490 Free PMC article.
-
DREADD-mediated amygdala activation is sufficient to induce anxiety-like responses in young nonhuman primates.Curr Res Neurobiol. 2023 Oct 5;5:100111. doi: 10.1016/j.crneur.2023.100111. eCollection 2023. Curr Res Neurobiol. 2023. PMID: 38020807 Free PMC article.
-
Widespread AAV1- and AAV2-mediated transgene expression in the nonhuman primate brain: implications for Huntington's disease.Mol Ther Methods Clin Dev. 2016 Jun 29;3:16037. doi: 10.1038/mtm.2016.37. eCollection 2016. Mol Ther Methods Clin Dev. 2016. PMID: 27408903 Free PMC article.
-
An Open Resource for Non-human Primate Optogenetics.Neuron. 2020 Dec 23;108(6):1075-1090.e6. doi: 10.1016/j.neuron.2020.09.027. Epub 2020 Oct 19. Neuron. 2020. PMID: 33080229 Free PMC article.
-
Titer and product affect the distribution of gene expression after intraputaminal convection-enhanced delivery.Stereotact Funct Neurosurg. 2014;92(3):182-94. doi: 10.1159/000360584. Epub 2014 Jun 12. Stereotact Funct Neurosurg. 2014. PMID: 24943657 Free PMC article.
References
-
- Gao G, Vandenberghe LH., and , Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. - PubMed
-
- Smith A, Collaco R., and , Trempe JP. Methods in Molecular Biology: AAV Vector Delivery to Cells in Culture. Vol. 246. Springer Verlag: Clifton, NJ; 2004. pp. 167–177. - PubMed
-
- Virella-Lowell I, Zusman B, Foust K, Loiler S, Conlon T, Song S, et al. Enhancing rAAV vector expression in the lung. J Gene Med. 2005;7:842–850. - PubMed
-
- Mueller C., and , Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

