Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion
- PMID: 20010940
- PMCID: PMC2816657
- DOI: 10.1038/sj.bjc.6605486
Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion
Abstract
Background: Epithelial-to-mesenchymal transition (EMT) is associated with decreased adhesion and acquisition of metastatic potential of breast cancer cells. Epithelial-to-mesenchymal transition is mediated, in part, by two transcription repressors, Snail and Slug, that are known to be targets of the Notch signaling pathway, and JAGGED1-induced Notch activation increases EMT. However, the events that lead to increased Notch activity during EMT of breast cancer cells are unknown.
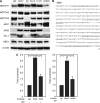
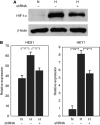
Methods: The accumulation of hypoxia inducible factors (HIFs) under hypoxia was detected by western blot analysis, and their effects on Notch signaling were measured by an in vitro Notch reporter assay. The expression of Notch target genes under hypoxia was tested by real-time PCR. The knockdown of HIF-1alpha was mediated by retroviral delivery of shRNA. The expression of Slug and Snail under hypoxia was measured by real-time PCR. Breast cancer cell migration and invasion under hypoxia were tested with cell migration and invasion kits.
Results: Hypoxia increased the expression of Notch target genes such as HES1 and HEY1 in breast cancer cells, as was expression of Notch receptors and ligands. The mechanism is likely to involve the accumulation of HIF-1alpha and HIF-2alpha in these cells by hypoxia, which synergised with the Notch co-activator MAML1 in potentiating Notch activity. Hypoxia inducible factor-1alpha was found to bind to HES1 promoter under hypoxia. Knockdown of HIF-1alpha with shRNA inhibited both HES1 and HEY1 expression under hypoxia. Hypoxia increased the expression of Slug and Snail, and decreased the expression of E-cadherin, hallmarks of EMT. Notch pathway inhibition abrogated the hypoxia-mediated increase in Slug and Snail expression, as well as decreased breast cancer cell migration and invasion.
Conclusion: Hypoxia-mediated Notch signaling may have an important role in the initiation of EMT and subsequent potential for breast cancer metastasis.
Figures


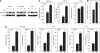

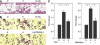
References
-
- Callahan R, Egan SE (2004) Notch signaling in mammary development and oncogenesis. J Mammary Gland Biol Neoplasia 9: 145–163 - PubMed
-
- Chen Y, De Marco MA, Graziani I, Gazdar AF, Strack PR, Miele L, Bocchetta M (2007) Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res 67: 7954–7959 - PubMed
-
- Dievart A, Beaulieu N, Jolicoeur P (1999) Involvement of Notch1 in the development of mouse mammary tumors. Oncogene 18: 5973–5981 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

