Design and synthesis of water-soluble bioconjugatable trans-AB-porphyrins
- PMID: 20011030
- PMCID: PMC2678728
- DOI: 10.1016/j.tet.2008.08.096
Design and synthesis of water-soluble bioconjugatable trans-AB-porphyrins
Abstract
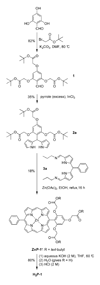
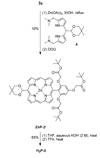
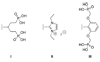
Three free base porphyrins have been prepared that bear a polar and facially encumbering 2,4,6-tris(carboxymethoxy)phenyl motif at one meso (5-) position. The only other substituent (15-position) comprises phenyl, formyl, or p-aminophenyl. The porphyrins exhibit solubility in water (or aqueous buffer solutions) at pH >/=7 and concentrations >1 mM at room temperature. The concise syntheses, water-solubility, and bioconjugatable handle make these porphyrin constructs suitable for biological applications.
Figures







Similar articles
-
Bioconjugatable porphyrins bearing a compact swallowtail motif for water solubility.Bioconjug Chem. 2006 May-Jun;17(3):638-53. doi: 10.1021/bc050337w. Bioconjug Chem. 2006. PMID: 16704201 Free PMC article.
-
A compact water-soluble porphyrin bearing an iodoacetamido bioconjugatable site.Org Biomol Chem. 2008 Jan 7;6(1):187-94. doi: 10.1039/b715072e. Epub 2007 Nov 23. Org Biomol Chem. 2008. PMID: 18075665
-
A new route to meso-formyl porphyrins.J Org Chem. 2004 Jul 23;69(15):5112-5. doi: 10.1021/jo049819b. J Org Chem. 2004. PMID: 15255746
-
Comparative study of binding interactions between porphyrin systems and aromatic compounds of biological importance by multiple spectroscopic techniques: A review.Spectrochim Acta A Mol Biomol Spectrosc. 2018 Jul 5;200:263-274. doi: 10.1016/j.saa.2018.04.037. Epub 2018 Apr 18. Spectrochim Acta A Mol Biomol Spectrosc. 2018. PMID: 29694930 Review.
-
Modifications of Porphyrins and Hydroporphyrins for Their Solubilization in Aqueous Media.Molecules. 2017 Jun 13;22(6):980. doi: 10.3390/molecules22060980. Molecules. 2017. PMID: 28608838 Free PMC article. Review.
Cited by
-
Molecular Catalyst Synthesis Strategies to Prepare Atomically Dispersed Fe-N-C Heterogeneous Catalysts.J Am Chem Soc. 2022 Oct 19;144(41):18797-18802. doi: 10.1021/jacs.2c08884. Epub 2022 Oct 10. J Am Chem Soc. 2022. PMID: 36215721 Free PMC article.
-
Structural control of the photodynamics of boron-dipyrrin complexes.J Phys Chem B. 2005 Nov 3;109(43):20433-43. doi: 10.1021/jp0525078. J Phys Chem B. 2005. PMID: 16853644 Free PMC article.
-
Synthesis of porphyrins bearing 1-4 hydroxymethyl groups and other one-carbon oxygenic substituents in distinct patterns.Tetrahedron. 2007 Oct 22;63(43):10657-10670. doi: 10.1016/j.tet.2007.07.108. Tetrahedron. 2007. PMID: 18037972 Free PMC article.
-
Bioconjugatable, PEGylated Hydroporphyrins for Photochemistry and Photomedicine. Narrow-Band, Red-Emitting Chlorins.New J Chem. 2016;40(9):7721-7740. doi: 10.1039/C6NJ01154C. Epub 2016 Jul 21. New J Chem. 2016. PMID: 28154477 Free PMC article.
-
Comparison of Kinetic Study and Protective Effects of Biological Dipeptide and Two Porphyrin Derivatives on Metal Cytotoxicity Toward Human Lymphocytes.Iran J Pharm Res. 2017 Summer;16(3):1059-1070. Iran J Pharm Res. 2017. PMID: 29201094 Free PMC article.
References
-
- Licha K. Top. Curr. Chem. 2002;222:1–29.
-
- Berg K, Selbo PK, Weyergang A, Dietze A, Prasmickaite L, Bonsted A, Engesaeter BØ, Angell-Petersen E, Warloe T, Frandsen N, Høgset A. J. Microscopy. 2005;218:133–147. - PubMed
-
- Kee HL, Nothdurft R, Muthiah C, Diers JR, Fan D, Ptaszek M, Bocian DF, Lindsey JS, Culver JP, Holten D. Photochem. Photobiol. 2008;84 - PubMed
-
- De Rosa SC, Brenchley JM, Roederer M. Nature Med. 2003;9:112–117. - PubMed
-
- Perfetto SP, Chattopadhyay PK, Roederer M. Nature Rev.: Immun. 2004;4:648–655. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
